Abstract
Introduction
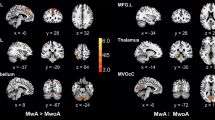
We investigated the diffusion-weighted MRI changes, apparent diffusion coefficient (ADC) values, and conventional MRI findings in specific brain areas during migraine attacks in patients with and without aura.
Methods
Included in the study were 22 patients (2 male, 20 female) aged between 17 and 49 years who were diagnosed as having migraine according to the diagnostic criteria of the International Headache Society. Also included in the study were 18 age- and sex-matched healthy volunteers. Hyperintense lesions were evaluated in conventional MR images. Heavily diffusion-weighted images, ADC maps, and segmented ADC maps generated for regional ADC (rADC) measurements, were also studied. ADC values from specific brain areas were used with appropriate region of interests (ROI).
Results
Migraine with aura was diagnosed in 13 patients and migraine without aura was diagnosed in 9 patients. A total of 23 hyperintense lesions within the periventricular white matter and deep white matter were detected in five patients (21.8%). All of these hyperintense lesions were seen in migraine patients with aura. In only one patient did a hyperintense lesion show an increased diffusion pattern on diffusion-weighted images and ADC maps. One hyperintense lesion was detected in the control group (5.5%). There was no significant difference in ADC values between the migraine and control groups.
Conclusion
T2-weighted hyperintense lesions were significantly more frequent in migraine patients especially in those with aura than in the control group. No diffusion alterations in diffusion-weighted images were detected in the infra- and supratentorial brain areas during migraine attacks in patients with and without aura.


Similar content being viewed by others
References
Headache Classification Committee of the International Headache Society (2004) Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 8(Suppl 7):1–96
Tzourio C, Tehindrazanarivelo A, Iglesias S, Alperovitch A, Chedru F, d’Anglejan-Chatillon J, Bousser MG (1995) Case-control study of migraine and risk of ischaemic stroke in young women. BMJ 310:830–833
Buring JE, Hebert P, Romero J, Kittross A, Cook N, Manson J, Peto R, Hennekens C (1995) Migraine and subsequent risk of stroke in the Physicians’ Health Study. Arch Neurol 52:129–134
Osborn RE, Alder DC, Mitchell CS (1999) MR imaging of the brain in patients with migraine headaches. AJNR Am J Neuroradiol 12:521–524
Rocca MA, Colombo B, Pratesi A, Comi G, Filippi M (2000) A magnetization transfer imaging study of the brain in patients with migraine. Neurology 54:507–509
van Buchem MA, Grossman RI, Armstrong C, Polansky M, Miki Y, Heyning FH, Boncoeur-Martel MP, Wei L, Udupa JK, Grossman M, Kolson DL, McGowan JC (1998) Correlation of volumetric magnetization transfer imaging with clinical data in MS. Neurology 50:1609–1617
Fazekas F, Koch M, Schmidt R, Offenbacher H, Payer F, Freidl W, Lechner H (1992) The prevalence of cerebral damage varies with migraine type: a MRI study. Headache 32:287–291
O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS (2001) Normal appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 57:2307–2310
Chabriat H, Pappata S, Poupon C, Clark CA, Vahedi K, Poupon F, Mangin JF, Pachot-Clouard M, Jobert A, Le Bihan D, Bousser MG (1999) Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke 30:2637–2643
Nusbaum AO, Tang CY, Wei T, Buchsbaum MS, Atlas SW (2000) Whole-brain diffusion MR histograms differ between MS subtypes. Neurology 54:1421–1427
Jager HR, Giffin NJ, Goadsby PJ (2005) Diffusion- and perfusion-weighted MR imaging in persistent migrainous visual disturbances. Cephalalgia 25:323–332
Oberndorfer S, Wober C, Nasel C, Asenbaum S, Lahrmann H, Fueger B, Grisold W (2004) Familial hemiplegic migraine: follow-up findings of diffusion-weighted magnetic resonance imaging (MRI), perfusion-MRI and [99mTc] HMPAO-SPECT in a patient with prolonged hemiplegic aura. Cephalalgia 24:533–539
Schocke MF, Seppi K, Esterhammer R, Kremser C, Jaschke W, Poewe W, Wenning GK (2002) Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology 26:575–580
Mascalchi M, Tessa C, Moretti M, Della Nave R, Boddi V, Martini S, Inzitari D, Villari N (2002) Whole brain apparent diffusion coefficient histogram: a new tool for evaluation of leukoaraiosis. J Magn Reson Imaging 15:144–148
Rovaris M, Filippi M, Calori G, Rodegher M, Campi A, Colombo B, Comi G (1997) Intra-observer reproducibility in measuring new putative MR markers of demyelination and axonal loss in multiple sclerosis: a comparison with conventional T2-weighted images. J Neurol 244:266–270
Schick S, Gahleitner A, Wober-Bingol C, Wober C, Ba-Ssalamah A, Schoder M, Schindler E, Prayer D (1999) Virchow-Robin spaces in childhood migraine. Neuroradiology 41:283–287
Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ (2004) Migraine as a risk factor for subclinical brain lesions. JAMA 291:427–434
Swartz RH, Kern RZ (2004) Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol 61:1366–1368
Kruit MC, Launer LJ, Ferrari MD, van Buchem MA (2006) Brain stem and cerebellar hyperintense lesions in migraine. Stroke 37:1109–1112
Gozke E, Ore O, Dortcan N, Unal Z, Cetinkaya M (2004) Cranial magnetic resonance imaging findings in patients with migraine. Headache 44:166–169
Soges LJ, Cacayorin ED, Petro GR, Ramachandran TS (1988) Migraine: evaluation by MR. AJNR Am J Neuroradiol 9:425–429
Cooney BS, Grossman RI, Farber RE, Goin JE, Galetta SL (1996) Frequency of magnetic resonance imaging abnormalities in patients with migraine. Headache 36:616–621
Fazekas F, Barkhof F, Filippi M (1998) Unenhanced and enhanced magnetic resonance imaging in the diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry 64(Suppl 1):S2–S5
Sanchez del Rio M, Bakker D, Wu O, Agosti R, Mitsikostas DD, Ostergaard L, Wells WA, Rosen BR, Sorensen G, Moskowitz MA, Cutrer FM (1999) Perfusion weighted imaging during migraine: spontaneous visual aura and headache. Cephalalgia 19:701–707
Tietjen GE, Al-Qasmi MM, Athanas K, Dafer RM, Khuder SA (2001) Increased von Willebrand factor in migraine. Neurology 57:334–336
Dreier JP, Kleeberg J, Petzold G, Priller J, Windmuller O, Orzechowski HD, Lindauer U, Heinemann U, Einhaupl KM, Dirnagl U (2002) Endothelin-1 potently induces Leao’s cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura? Brain 125:102–112
Olesen J, Larsen B, Lauritzen M (1981) Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 9:344–352
Woods RP, Iacoboni M, Mazziotta JC (1994) Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med 331:1689–1692
Cutrer FM, Sorensen AG, Weisskoff RM, Ostergaard L, Sanchez del Rio M, Lee EJ, Rosen BR, Moskowitz MA (1998) Perfusion-weighted imaging defects during spontaneous migrainous aura. Ann Neurol 43:25–31
Seto H, Shimizu M, Futatsuya R, Kageyama M, Wu Y, Kamei T, Shibata R, Kakishita M (1994) Basilar artery migraine. Reversible ischemia demonstrated by Tc-99m HMPAO brain SPECT. Clin Nucl Med 19:215–218
Andersen AR, Friberg L, Olsen TS, Olesen J (1988) Delayed hyperemia following hypoperfusion in classic migraine. Single photon emission computed tomographic demonstration. Arch Neurol 45:154–159
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 98:4687–4692
Woessner DE (1963) NMR spin-echo self diffusion measurements on fluids undergoing restricted diffusion. J Phys Chem 67:1365–1367
Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D (1990) Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 176:439–445
Chabriat H, Vahedi K, Clark CA, Poupon C, Ducros A, Denier C, Le Bihan D, Bousser MG (2000) Decreased hemispheric water mobility in hemiplegic migraine related to mutation of CACNA1A gene. Neurology 54:510–512
Butteriss DJ, Ramesh V, Birchall D (2003) Serial MRI in a case of familial hemiplegic migraine. Neuroradiology 45:300–303
Lindahl AJ, Allder S, Jefferson D, Allder S, Moody A, Martel A (2002) Prolonged hemiplegic migraine associated with unilateral hyperperfusion on perfusion weighted magnetic resonance imaging. J Neurol Neurosurg Psychiatry 73:202–203
Cercignani M, Inglese M, Pagani E, Comi G, Filippi M (2001) Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am J Neuroradiol 22:952–958
Rocca MA, Colombo B, Inglese M, Codella M, Comi G, Filippi MA (2003) Diffusion tensor magnetic resonance imaging study of brain tissue from patients with migraine. J Neurol Neurosurg Psychiatry 74:501–503
Rocca MA, Ceccarelli A, Falini A, Tortorella P, Colombo B, Pagani E, Comi G, Scotti G, Filippi M (2006) Diffusion tensor magnetic resonance imaging at 3.0 tesla shows subtle cerebral grey matter abnormalities in patients with migraine. J Neurol Neurosurg Psychiatry 77:686–689
Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC (1995) Brain stem activation in spontaneous human migraine attacks. Nat Med 1:658–660
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00234-007-0217-1
Rights and permissions
About this article
Cite this article
Degirmenci, B., Yaman, M., Haktanir, A. et al. Cerebral and cerebellar ADC values during a migraine attack. Neuroradiology 49, 419–426 (2007). https://doi.org/10.1007/s00234-006-0201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0201-1