Abstract
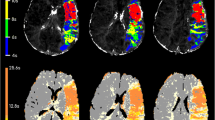
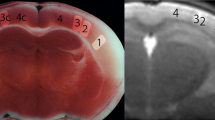
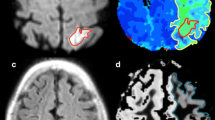
Diffusion and perfusion weighted MRI have been widely used in ischaemic stroke. We studied 17 patients in whom ischaemic areas showed an ischaemic core, an area of infarct growth and hypoperfused but ultimately surviving tissue. Apparent diffusion coefficients (ADC) were measured on days 1, 2, and 8 in the three subregions and in contralateral control areas. Cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) were measured in these regions on day 1 perfusion maps. On day 1, the ischaemic core had very low ADC and CBF and increased MTT. The ADC in the ischaemic core gradually increased during the week. The area of infarct growth on day 1 had slightly but significantly decreased ADC (96% of control, P=0.028), moderately decreased CBF and increased MTT. On day 1 the hypoperfused but surviving tissue had slightly but significantly increased ADC (103% of control, P=0.001), mildly decreased CBF and increased CBV and MTT. The ADC of the area of infarct growth decreased to the same level as in the ischaemic core on days 2 and 8. That of surviving tissue was still above normal on day 2 (103% of control), but had returned to the normal level by day 8. Measurement of ADC combined with perfusion MRI may help distinguish different subregions in acutely hypoperfused brain.


Similar content being viewed by others
References
Moseley ME, Cohen Y, Mintorovitch J, et al (1990) Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med 14: 330–346
Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA (1995) Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab 15: 1002–1011
Pierpaoli C, Righini A, Linfante I, Tao-Cheng JH, Alger JR, Di Chiro G (1993) Histopathologic correlates of abnormal water diffusion in cerebral ischemia: diffusion-weighted MR imaging and light and electron microscopic study. Radiology 189: 439–448
Knight RA, Dereski MO, Helpern JA, Ordidge RJ, Chopp M (1994) Magnetic resonance imaging assessment of evolving focal cerebral ischemia. Comparison with histopathology in rats. Stroke 25: 1252–1261
Østergaard L, Johannsen P, Host-Poulsen P, et al (1998) Cerebral blood flow measurements by magnetic resonance imaging bolus tracking: comparison with [15O]H2O positron emission tomography in humans. J Cereb Blood Flow Metab 18: 935–940
Østergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magn Reson Med 36: 726–736
Baird AE, Benfield A, Schlaug G, et al (1997) Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol 41: 581–589
Fisher M, García JH (1996) Evolving stroke and the ischemic penumbra. Neurology 47: 884–888
Karonen JO, Vanninen RL, Liu Y, et al (1999) Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke. Ischemic penumbra predicts infarct growth. Stroke 30: 1583–1590
Le Bihan D (1990) Diffusion/perfusion MR imaging of the brain: from structure to function. Radiology 177: 328–329
Wu O, Koroshetz WJ, Ostergaard L, et al (2001) Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke 32: 933–942
Oppenheim C, Grandin C, Samson Y, et al (2001) Is there an apparent diffusion coefficient threshold in predicting tissue viability in hyperacute stroke? Stroke 32: 2486–2491
Rohl L, Ostergaard L, Simonsen CZ, et al (2001) Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke 32: 1140–1146
Parsons MW, Yang Q, Barber PA, et al (2001) Perfusion magnetic resonance imaging maps in hyperacute stroke: relative cerebral blood flow most accurately identifies tissue destined to infarct. Stroke 32: 1581–1587
Østergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 36: 715–725
Liu Y, Karonen JO, Vanninen RL, et al (2000) Cerebral hemodynamics in human acute ischemic stroke: a study with diffusion- and perfusion-weighted magnetic resonance imaging and SPECT. J Cereb Blood Flow Metab 20: 910–920
Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME (1999) Evaluation of early reperfusion and i.v. tPA therapy using diffusion- and perfusion-weighted MRI. Neurology 52: 1792–1798
Fiehler J, Foth M, Kucinski T, et al (2002) Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke 33: 79–86
Li F, Liu KF, Silva MD, et al (2000) Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke 31: 946–954
Furlan M, Marchal G, Viader F, Derlon JM, Baron JC (1996) Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol 40: 216–226
Marchal G, Beaudouin V, Rioux P, et al (1996) Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke 27: 599–606
Marchal G, Benali K, Iglesias S, Viader F, Derlon JM, Baron JC (1999) Voxel-based mapping of irreversible ischaemic damage with PET in acute stroke. Brain 122: 2387–2400
Schlaug G, Benfield A, Baird AE, et al (1999) The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 53: 1528–1537
Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, Marks MP, Albers GW (2001) Relationship between severity of MR perfusion deficit and DWI lesion evolution. Neurology 57: 1205–1211
Jones TH, Morawetz RB, Crowell RM, et al (1981) Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg 54: 773–782
Ernst T, Chang L, Itti L, Speck O (1999) Correlation of regional cerebral blood flow from perfusion MRI and SPECT in normal subjects. Magn Reson Imaging 17: 349–354
Rempp KA, Brix G, Wenz F, Becker CR, Guckel F, Lorenz WJ (1994) Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 193: 637–641
Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D (1984) Regional cerebral blood flow and oxygen consumption in human aging. Stroke 15: 635–641
Miyabe M, Mori S, van Zijl PC, et al (1996) Correlation of the average water diffusion constant with cerebral blood flow and ischemic damage after transient middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab 16: 881–891
Pérez-Trepichio AD, Xue M, Ng TC, et al (1995) Sensitivity of magnetic resonance diffusion-weighted imaging and regional relationship between the apparent diffusion coefficient and cerebral blood flow in rat focal cerebral ischemia. Stroke 26: 667–675
Pierce AR, Lo EH, Mandeville JB, González RG, Rosen BR, Wolf GL (1997) MRI measurements of water diffusion and cerebral perfusion: their relationship in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab 17: 183–190
Yonas H, Pindzola RR (1994) Physiological determination of cerebrovascular reserves and its use in clinical management. Cerebrovasc Brain Metab Rev 6: 325–340
Ulug AM, Beauchamp N Jr, Bryan RN, van Zijl PC (1997) Absolute quantitation of diffusion constants in human stroke. Stroke 28: 483–490
Helenius J, Soinne L, Perkio J, et al (2002) Diffusion-weighted MR imaging in normal human brains in various age groups. AJNR 23: 194–199
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168: 497–505
Le Bihan D (1988) Intravoxel incoherent motion imaging using steady-state free precession. Magn Reson Med 7: 346–351
Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J (1990) Echo-planar imaging of intravoxel incoherent motion. Radiology 177: 407–414
Wirestam R, Borg M, Brockstedt S, Lindgren A, Holtas S, Stahlberg F (2001) Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique. Acta Radiol 42: 123–128
Marchal G, Furlan M, Beaudouin V, et al (1996) Early spontaneous hyperperfusion after stroke. A marker of favourable tissue outcome? Brain 119: 409–419
Desmond PM, Lovell AC, Rawlinson AA, et al (2001) The value of apparent diffusion coefficient maps in early cerebral ischemia. AJNR 22: 1260–1267
Acknowledgements
This work was supported by Kuopio University Hospital (EVO funding 307/97 and 21/98), the Radiological Society of Finland, the Academy of Finland, the Sigrid Jusélius Foundation, the Instrumentarium Science Foundation, Biomedicum Helsinki Foundation, the Aarne Koskelo Foundation, the Paulo Foundation, and the Paavo Nurmi Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Karonen, J.O., Vanninen, R.L. et al. Detecting the subregion proceeding to infarction in hypoperfused cerebral tissue: a study with diffusion and perfusion weighted MRI. Neuroradiology 45, 345–351 (2003). https://doi.org/10.1007/s00234-003-0959-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-003-0959-3