Abstract
Glycosaminoglycans (GAGs) are essential components of the extracellular matrices (ECMs) located on the outer surface of cellular membranes. They belong to the group of polysaccharides involved in diverse biological processes acting on the surface and across natural lipid membranes. Recently, particular attention has been focused on possible role of GAGs in the amyloid deposits. The amyloid formation is related to a disorder in protein folding, causing that soluble—in normal conditions—peptides become deposited extracellularly as insoluble fibrils, impairing tissue structure and its function. One of the hypothesis holds that GAGs may inhibit amyloid formation by interacting with the lipid membrane by blocking the accumulation of protein aggregates on the membrane surface. Although the biophysical properties of GAGs are described rather well, little is known about the nature of association between these polysaccharides and components of natural cell membranes. Therefore, a study of GAGs influence on membrane lipids is of particular importance. The aim of the present work is to get insight into the effect of hydrophilic dextran sulfate (DS)—that can be considered as GAG analogue—on membrane lipids organization. This study was based on examining interactions between DS sodium salt of molecular weight equal to about 40 kDa (DS40), dissolved in water subphase, and a model membrane, mimicked as Langmuir monolayer, formed by representative natural membrane lipids: cholesterol and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) as well as their mixtures. Due to the fact that calcium ions in excess may accumulate in the lipid membrane, attracting high molecular weight molecules to their surface, the influence of calcium ions present in the subphase on the DS40 activity has also been examined. It has been found that negatively charged DS, forming a sublayer underneath the monolayer, barely interacts with membrane lipids; however, in the presence of calcium ions the electrostatic interactions between DS40 and lipid membrane are significantly enhanced, leading to the formation of network-like crystalline structures at the surface of model membrane, which can prevent incorporation and interaction with other extracellular molecules, e.g., proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycosaminoglycans (GAGs) are essential components of the extracellular matrices (ECMs) that contribute to the stability, development, and communication of natural cells within all kinds of tissues (Bosman and Stamenkovic 2003; Saridaki et al. 2012). GAGs belong to the group of polysaccharides composed of long unbranched carbohydrate chains with repeating disaccharide units of an amino sugar (either N-acetylglucosamine or N-acetylgalactosamine) along with an uronic acid sugar (either glucuronic acid or iduronic acid) or galactose (Wight et al. 1991; Ruponen et al. 1999; Papy-Garcia et al. 2011). Although molecular structures of GAGs that naturally occur in living cells are rather uncomplicated, these polysaccharides can be subjected to further transformations, including enzymatic sulfonation of hydroxyl groups in carbohydrate moieties. As a result, a number of GAGs derivatives can be formed; both sulfated (including heparin and heparan sulfate, keratan sulfate, chondroitin sulfate, and dermatan sulfate) and non-sulfated (e.g., hyaluronic acid) (Lindahl and Li 2009; Papy-Garcia et al. 2011).
GAGs are primarily located on the outer surface of the cell membrane, being covalently attached to core proteins, forming proteoglycans (Sahoo and Schwille 2013). In smaller amounts, GAGs can also be found in the extracellular matrixes (ECMs) as free macromolecules. Hyaluronic acid—being an exception—does not form covalent linkages with membrane proteins, but interacts non-covalently with other ECMs molecules. The binding properties of the GAG chains are mainly determined by the substitution with the sulfo groups (Laabs et al. 2005), which results in highly negative charged structures. Therefore, GAGs are considered to be the strongest natural polyanions. Highly negative charge of the GAG chains induces binding of cations present within the ECM, what additionally ensures sufficient hydration and proper hydrostatic pressure inside tissues (Abaterusso and Gabaro 2006; Fukamil et al. 2007).
GAGs are involved in diverse biological processes acting on the surface and across natural cell membranes (Ruponen et al. 1999; Uygun et al. 2009; Papy-Garcia et al. 2011). Various scientific reports indicate a wide range of GAG application in the field of gene transfer. Recently, a lot of attention has been devoted to develop new methods in order to introduce genetic material into natural cells. On one hand, polyanionic GAGs interfere with gene transfer by binding to the positively charged complexes (e.g., DNA complexes). The resulting linkages can lead to changes in molecular charge and size of such complexes, affecting the cellular uptake. It was reported that GAGs, due to their ability to interact with cationic lipids, can bind to liposome/DNA complexes and thus initiate the release of DNA from the liposomes. On the other hand, GAGs mediate the binding of the cationic complexes to the surface of biological cells and in this way may act as important receptors for the cellular entry of the gene transfer complexes (Mislick and Baldeschwieler 1996; Ruponen et al. 1999).
GAGs also appear to play a significant role in the amyloid deposits in human pathologies (Ancsin 2003; Alexandrescu 2005; Smits et al. 2010; Saridaki et al. 2012). The amyloid formation is related to a protein folding disorder, which results in extracellular deposition of normally soluble peptides as insoluble fibrils, leading to the impaired tissue structure and its function.
Although GAGs seem to play an active role due to their high content in the extracellular matrix as well as their ability to interact with different types of proteins, their precise mode of action and induced effects still remain under debate. One of the hypotheses assumes that GAGs may act as pathological chaperones, which induce formation and stabilization of amyloid fibril. GAGs binding to amyloid fibrils, mainly through electrostatic interactions involving the negative polysaccharide charges and positively charged protein residues, may lead to abnormal accumulation of amyloids. This may result in the development of amyloidosis, being related—in brain—to the pathology of various neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and other prion-related diseases (Ruponen et al. 1999; Capila and Linhardt 2002; Bulow and Hobert 2004; Laabs et al. 2005). However, contradictory hypothesis implies that GAGs can inhibit amyloid formation by blocking the interactions of protein aggregates with cell membranes. Recent studies have shown that heparin does not directly bind or alter the structural and morphological properties of protein fibrils, pointing to its interaction with the cell surface. It is suggested that GAGs interact with natural cell membrane, probably competing with protein aggregates for the same binding sites on the cell surface (Saridaki et al. 2012; Lannuzzi et al. 2015). Taking into consideration that the presence of GAGs may significantly affect the structure and dynamics of biological cell membranes, it seems crucial to examine the potential impact of GAGs on membrane lipids.
The chemical complexity of the GAGs not only makes them less sensitive to conventional methods of analysis, but also leads to limitations in their isolation as well as fractionation. Thus, even fractionated and purified samples may contain a heterogeneous mixture of sequences of different lengths and compositions (Hallak et al. 2007). Considering these limitations, the analogues of GAGs, that can be obtained commercially in various average molecular sizes are often used instead of GAGs. Due to certain structural similarities, dextran as well as its derivatives can successfully replace cellular GAGs in experiments on model lipid membranes (Santos et al. 2005a, b, 2007a, b; Peetla et al. 2009). Most commonly applied dextran sulfate (DS) is an artificially sulfated polysaccharide produced by growing of Leuconostoc mesenteroides bacterial cultures. DS contains a high percentage of consecutive α(1,6)-glucosidic linkages and a relatively low percentage of α(1,3)-linked residues that makes the molecule stable and highly resistant to digestive enzymes, especially α-amylases, present in human body.
Dextran and its derivatives, considered as GAG analogues, were successfully applied to investigate various aspects of cellular interactions in lipid membranes. It has been shown that physicochemical properties of model GAGs can significantly influence the interactions with natural lipids as well as they can either increase or decrease the efficiency of drug delivery through the cellular membrane (Costin and Barnes 1975; Ross et al. 2001). On one hand, these rigid polysaccharides have the ability to inhibit undulation of lipid membranes. Experiments carried out on model black lipid membranes (BLMs) (Diederich et al. 1999) demonstrated that dextran derivative attached to the BLM surface significantly changes its mechanical properties. As a result, the increase of membrane viscosity and reduction of its stability was observed. On the other hand, model GAGs may decrease the energy barrier necessary to form a pore within lipid membrane, either by increasing the surface tension of the membrane or by reducing the edge energy of the pore (Diederich et al. 1999).
Intensive research on dextran and its derivatives—as model GAGs—has been found effective in the treatment of viral infections, in particular Sendai or HIV viruses, by inhibiting the entry of viruses into cells (Ramalho-Santos and Pedroso de Lima 2001; Wiethoff et al. 2001; Ruponen et al. 2004; Naessens et al. 2005). Moreover, these polysaccharides also seem to be promising in preventing the development of atherosclerosis in arteries and heart diseases (Nauck and Rifai 2000; Simons et al. 2002).
Although the biophysical properties of DS have been studied rather well, the nature of its affinity to natural membranes has not been fully elucidated. Therefore, the aim of the present work is to examine the behavior of water-soluble DS of molecular weight equal to about 40 kDa (DS40) (Fig. 1) on model lipid environment. Since natural membranes are complex entities, in order to get insight into their interaction with biomolecules, model systems are usually applied, which are well-defined and simplified structures of natural membranes, and enable to study a particular aspect of biomolecule-membrane component interactions. In this respect, Langmuir monolayers are one of the most popular and successful membrane models (Maget-Dana 1999; Stefaniu et al. 2014; Nobre et al. 2015). With Langmuir monolayers, it is possible to easily construct a membrane of interest. The simplest model is a one-component monolayer, usually formed by one of the major membrane lipids, such as cholesterol, 1,2-dipalmitoyl-sn-3-phosphatidylcholine (DPPC) or other membrane component of interest. Such a model is certainly an oversimplification; however, it is useful in some cases, for example, it enables an easy verification of which of the membrane components are important in a particular aspect of its biological activity. More realistic are multicomponent Langmuir monolayers as membrane-mimicking systems, prepared by mixing of the representative components in an appropriate proportion found in natural systems. As the simplest model, mixed cholesterol/DPPC monolayer, mimicking an eukaryotic plasma membrane, is usually applied.
Taking the above into consideration, in this paper, we have studied the interactions between DS, penetrating into artificial membrane, mimicked as Langmuir monolayer, composed of representative lipids of mammalian cell membrane, such as DPPC, cholesterol, and their mixture. Since the increase of ionic strength or introduction of divalent cations are known to reduce the electrostatic repulsive forces between cell surfaces and thus may affect the interaction of DS40 with membrane lipids [which has been observed in vivo, showing that calcium ions in excess can accumulate at the lipid membrane, attracting high molecular weight molecules to its surface (Anderson et al. 1977)], the influence of Ca2+ on the DS40 activity has also been examined. The concentration of calcium ions used in our experiments (3 mmol dm−3) corresponds to its physiological content in the ECM.
Experimental
Materials
Cholesterol (> 99%) and DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine, synthetic, > 99%) were used as typical biomembrane lipids. Initially, each lipid was dissolved in a spectroscopic grade chloroform/ethanol (4:1 v/v) mixture made from chloroform (dedicated for HPLC, ≥ 99%) and anhydrous ethanol (> 99%). Then, the prepared solutions were spread onto the aqueous subphase in order to form insoluble films at the air/water interface. Stock solutions of cholesterol and DPPC were also applied to form mixed monolayers with different molar fractions of both constituents. Ultrapure water (from a Millipore system) and 4 × 10−3 mmol dm−3 solution of DS sodium salt with molecular weight of about 40 kDa (DS40) as well as DS40 solution enriched with calcium chloride (of the concentration 3 mmol dm−3) were used as subphases.
Cholesterol, DPPC, and DS40 were purchased from Sigma–Aldrich and used without further purification, while 3 mmol dm−3 calcium chloride aqueous solution was prepared from 0.05 mol dm−3 stock solution, manufactured by POCh (Poland).
Methods
Langmuir Monolayer Technique
The surface pressure–area (π/A) isotherms were recorded by using a one-barrier Langmuir trough (NIMA, UK) of 300 cm2 total area. Surface pressure was measured with accuracy of ± 0.1 mN m−1 using a Wilhelmy plate made of ashless chromatography paper (Whatman Chr1). Before each measurement, water subphase was cleaned by closing the barrier and aspirating water until surface pressure readings were not exceeding ± 0.1 mN m−1, in comparison with values of surface pressure detected with the opened barrier. The subphase temperature was controlled thermostatically, by a circulating water system (Julabo), and kept at 20 °C ± 0.1 °C. Spreading solutions were deposited drop by drop onto the water subphase with a 250 µl Hamilton microsyringe, precise to 5.0 µl. After spreading, monolayers were left to equilibrate for 10 min and then compressed with barrier speed of 20 cm2 min−1. Each recorded π/A isotherm was repeated at least twice to ensure high reproducibility of the results.
Brewster Angle Microscopy (BAM)
Textures of the selected Langmuir monolayers were visualized with Brewster angle microscope, BAM (Accurion GmbH, Germany), equipped with a 50 mW laser emitting p-polarized light at a wavelength of 658 nm and an objective with tenfold magnification. The microscope was installed over a KSV NIMA Langmuir trough (Finland) with two barriers and 841 cm2 of its total area. BAM images show selected fragments of Langmuir monolayers with dimensions of 360 µm times 200 µm.
Langmuir–Blodgett Deposition Technique (LB)
Langmuir monolayers were transferred onto a solid surface (ruby muscovite mica of V-1 quality, purchased from Continental Trade), using LB technique. Before each experiment, mica substrate was placed, respectively, in the following subphases: water, aqueous solution of DS40 and DS40-containing Ca2+. After spreading solution of pure DPPC onto subphase, the monolayer was left to equilibrate for 10 min and then compressed to constant values of surface pressure (20 mN m−1). LB deposition was carried out by lifting-off the mica substrate from the relevant subphase through the monolayer with a dipper speed of 1 mm min−1 .
Atomic Force Microscopy (AFM)
Topographic images of the transferred films were recorded in air under ambient conditions using AFM (Agilent 5500) working in non-contact mode. The Al-coated force modulation silicon probes (Nanosensors) with spring constant about 2 N m−1, resonant frequencies about 80 kHz, and the tip radius equal to 7 nm were used. The set point and all gains were adjust to obtain minimal noise and high-quality images of examined surfaces. The topography images of each sample were recorded in several arbitrarily chosen locations. AFM data analysis was carried out using WSxM free software (Horcas et al. 2007).
Results and Discussion
In order to determine the effect of DS40 on model cellular membrane, in the first step of our investigations, we have examined its influence separately on two major membrane lipids: cholesterol and DPPC, and subsequently on cholesterol/DPPC mixed monolayers as a simplified biomembrane model. Although the behavior of the above membrane lipids and their mixtures have already been studied in detail (Cadena-Nava et al. 2006; Sabatini et al. 2008; Miñones et al. 2009), we present the π/A isotherms and BAM images recorded on water subphase for the purpose of comparison with the results obtained for DS40 and/or Ca2+-containing subphases.
π/A Isotherms and BAM Images for Pure Lipids Monolayers on Different Subphases
Surface pressure–area (π/A) isotherms registered for pure cholesterol monolayers formed on different subphases at 20 °C, complemented with the values of compression moduli (Cs−1), illustrated as a function of surface pressure (π), complemented with selected BAM images are presented in Fig. 2.
The results recorded for water subphase are in good agreement with those presented elsewhere (Cadena-Nava et al. 2006). The course of the obtained π/A isotherms indicates that cholesterol forms a highly condensed monolayer in the form of a typical solid film (S) on all the applied subphases, which can be also confirmed by high compression moduli values (called also “surface elasticity”, defined as \({C_s}^{{ - 1}}=A{\left( {\partial \uppi /\partial A} \right)_{\text{T}}}\) ; A denotes average area per lipid molecule in a monolayer) (Davies and Rideal 1963) (Fig. 2). The introduction of DS40 and/or Ca2+ to the subphase practically does not affect both the shape and the position of the isotherm from cholesterol. The observed difference in the collapse pressure (πc) value for cholesterol monolayer spread on DS40+Ca2+ (47 mN m−1) as compared to other investigated here subphases (πc ~ 45 mN m−1) is negligible. The visualization of cholesterol monolayer with BAM (Fig. 2) does not reveal any significant differences in the films texture on different subphases investigated. Therefore, it can be concluded that the introduction of DS40 and/or Ca2+ into water subphase has no impact on the organization as well as packing arrangement of sterol molecules. For comparison, similar experiments were performed with another important membrane lipid: DPPC (Fig. 3).
The shape of π/A isotherm registered for pure DPPC monolayer on the water subphase is very similar to that recorded on aqueous DS40 or Ca2+ solutions, with a characteristic plateau at 5 mN m−1, which have been ascribed to a phase transition between liquid-expanded and liquid-condensed (LE–LC) states (Miñones et al. 2009). This plateau is visible as a minimum on Cs−1 versus π plots for pure DPPC monolayer formed both on water, water-containing Ca2+, and aqueous DS40 solution (Fig. 3, inset). A striking difference is the disappearance of plateau region for DPPC on DS40-containing Ca2+ solution. This is associated with monolayer condensation as evidenced by higher Cs−1 values as compared to DPPC films spread on other investigated solutions. Moreover, it is worth noticing that stability of films formed on DS40-containing subphases increases as shown by higher collapse pressure values.
Despite close similarities between π/A isotherm registered for DPPC monolayer on water and on aqueous Ca2+ or DS40 solution, there are visible differences in the morphology of the studied systems (Fig. 4). Accurate analysis of films textures with BAM shows changes in the shape of domains formed at the surface, particularly well distinguishable just above the plateau region (~ 12 mN m−1). Namely, for DPPC monolayers spread on water, the domains show characteristic ‘clover’ shape, while upon addition of Ca2+ ions their shape is changed to the ‘flower’-like, which is in agreement with literature (Camara and Wilke 2017). On aqueous DS40 solution domains morphology is changed to the ‘S’-shaped structures. It is well known that DPPC molecules in a broad pH range (2.1–13.9) occur in the electroneutral (zwitterionic) form (Fisar 2005). This means that at physiological pH of the extracellular space (7.35–7.45) phospholipid molecules comprise an equal number of ionized groups with opposite charge. The negative charge is localized on the oxygen atom of the phosphate group, while a positive one—on the nitrogen atom of the choline group. Stability of DPPC monolayer is maintained by lateral repulsions between film molecules. Upon introduction of calcium ions, the negatively charged phosphate groups of DPPC molecules interact electrostatically with Ca2+, turning the zwitterionic monolayer to cationic, without any pronounced changes in the isotherm characteristics. For DPPC spread on DS40 solution, the polyelectrolyte brings in an additional negative charge due to the formation of a sublayer of DS40 strains underneath the phospholipid film as proved elsewhere (Santos et al. 2005b; Camara and Wilke 2017). This negative charge is reduced by the introduction of calcium ions into aqueous dextran sulfate solution as bivalent ions contribute to electrostatic interactions between the anionic phosphate groups of DPPC and the anionic polysulfate groups of DS40 by the formation of calcium bridges. In consequence, DPPC/Ca2+/DS40 complex is formed, resulting in strong adsorption of DS40 to the surface, already confirmed in literature (Santos et al. 2005b), and condensation of DPPC monolayer, which is reflected both in higher Cs−1 values and disappearance of liquid-expanded to liquid-condensed transition. In consequence, in BAM images characteristic for this region domains are replaced with network-like crystalline structures.
In order to characterize the observed structures in detail, we have transferred DPPC/Ca2+/DS40 complex to solid support, using the LB technique. For comparison, DPPC monolayers spread on water and aqueous DS40 solution have also been transferred. The obtained AFM images deposited on mica substrates are presented in Fig. 5a–c.
AFM images of a DPPC monolayer transferred from a water subphase, b DPPC film transferred from an aqueous solution of DS40, and c coupled DPPC/Ca2+/DS40 complex, deposited on mica substrates at the surface pressure of 20 mN m−1 using LB technique, complemented with height profiles from highlighted lines (d, e, and f, respectively)
The AFM image of DPPC monolayer transferred from water subphase (Fig. 5a) shows uniform and homogenous structure with minimal height or compliance variations as reflected by the root mean square (RMS) roughness parameter equal to 0.14 nm. Similar structure for DPPC monolayer deposited on mica substrate has already been presented in literature (Kim et al. 2013). The surface of DPPC film transferred from an aqueous DS40 solution is characterized by a slightly higher RMS roughness parameter reaching up to 0.3 nm; however, the homogeneity of the observed surface is preserved (Fig. 5b). The AFM image of DPPC/Ca2+/DS40 complex (Fig. 5c) shows continuous layer with clearly visible gaps (RMS parameter equal to 0.37 nm), confirming the formation of complex, which structure was found to depend on the amount of adsorbed DS40 (Huster 1998).
π/A Isotherms for Cholesterol/DPPC Mixed Monolayers on DS-Containing Subphases
π/A isotherms for cholesterol/DPPC mixed monolayers, formed on different subphases at 20 °C, are presented in Fig. 6a–c. Although this system, considered as the simplest model of cell membrane, has been widely investigated (see for example: Wydro and Hąc-Wydro 2007), the monolayer results obtained on water are included in this paper for the purpose of comparison with those obtained on DS-containing subphases.
It is seen that upon increasing of cholesterol content in DPPC monolayers for all the studied subphases, the isotherms are shifted towards lower values of the average area per lipid molecule (proving the so-called “condensing effect” of cholesterol). Such a behavior of area condensation is typical for phospholipid–cholesterol mixtures and has been widely described in literature (Sabatini et al. 2008).
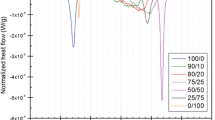
The comparison of maximum values of the compressibility moduli (Cs−1), presented in Fig. 7, clearly demonstrates that the change of water subphase to DS40 solution practically does not influence their elasticity, while upon the addition of calcium ions the monolayers become more rigid (higher Cs−1 values).
To get insight into the interactions on different subphases, the plots of the average area per molecule (A12) in mixed monolayers as a function of cholesterol molar fraction (at different constant values of surface pressures: 10, 15, 20, 25, 30 mN m−1) were drawn from experimental data points of isotherms (Fig. 8). The obtained curves, presented in Fig. 8a–c, allow to conclude about interactions and miscibility of the film components. If either an ideal mixed monolayer is formed or two components are completely immiscible, the additivity rule is obeyed (Dynarowicz-Latka and Kita 1999):
where A1, A2 are defined as the average area per lipid molecule in pure monolayers and X1, X2 correspond to their molar fractions in mixed film, and the plots presenting the average area per lipid molecule as a function of molar fraction form a straight line (dotted lines in Fig. 8a–c). Any deviations from the straight indicate miscibility and non-ideality. Considering the results for cholesterol/DPPC system, apparent deviations from ideality are observed. This clearly proves the miscibility of cholesterol and DPPC in monolayers spread on all the studied subphases.
For miscible system, it is possible to perform quantitative analysis of interactions, which can be done with the values of the excess free enthalpy of mixing (ΔGex) plotted as a function of cholesterol molar fraction (at different constant values of surface pressure: 10, 15, 20, 25, 30 mN m−1), calculated according to the following Eq. (2) (Gaines 1966; Costin and Barnes 1975):
wherein N is the Avogadro number.
The obtained dependencies, presented in Fig. 9a–c, show that the strength of cholesterol–DPPC interactions depends on the kind of the applied subphase.
Considering films formed on water, negative values of ΔGex are observed, indicating the existence of strong interactions between film-forming molecules. The minimum value of ΔGex corresponds to the mixture of the strongest interactions and maximum thermodynamic stability, which occurs at Xchol ~ 0.5 (Wydro and Hąc-Wydro 2007). In literature, this phenomenon has been interpreted as being due to stable cholesterol–DPPC complex formation of 1:1 stoichiometry (Brzozowska and Figaszewski 2002; Dynarowicz-Latka et al. 2002; Sabatini et al. 2008) due to the hydrogen bond formation between molecules. Upon introducing of DS40 into the subphase, the strength of interactions remains similar as evidenced by comparable maximum ΔGex values on both subphases. However, the additionally introduced calcium ions have a considerable impact on DS40 interaction with model lipid membrane, which is seen in less negative ΔGex values (that change from −1 kJ mol−1 at the minimum for water/DS40 solution to – 0.5 kJ mol−1 for DS40 + Ca2+). From the thermodynamic point of view this means that bivalent ions exert unfavorable effect on DPPC/cholesterol monolayers on DS40 solution. Such thermodynamic destabilization of the monolayers results from weakening of the DPPC–sterol interaction by the formation of calcium bridges between DPPC and DS40. Moreover, additional effect of conformational changes in the presence of calcium ions of the phosphatidylcholine headgroup, which has been reported (Simon et al. 1975; Akutsu and Seelig 1981; Ross et al. 2001), can also account to the magnitude of interactions.
Conclusions
The results of our experiments on the effect of DS or/and Ca2+ ions on major membrane lipid monolayers show no effect on cholesterol while pronounced changes have been observed for DPPC monolayer. Both plateau transition and elasticity of monolayer are altered in the presence of divalent ions in the DS subphase, accompanied by significant changes in the morphology of monolayer, showing network-like crystalline structures, associated with the formation of calcium bridges between phosphate groups of DPPC and polysulfate groups of DS. The results of our experiments also evidenced that DS in the presence of Ca2+ has a significant influence on model biological membrane. Hydrogen bonds between cholesterol and DPPC in mixed monolayers are weakened in the presence of Ca2+, due to electrostatic interactions with negatively charged groups of DPPC and DS. In this way, DPPC–cholesterol monolayers are destabilized as proved with less negative ΔGex values. Moreover, they lose their flexibility and become more rigid. Therefore, the formation of calcium bridges can be of biological importance as their presence prevents DS from interacting with other extracellular molecules, for example, proteins, and hinders the possibility of incorporation of other solutes. Strong interactions that occur between DS and membrane lipids via calcium ions may also be of great importance in discussing a possible role of ECMs for lipid dynamics in cell membranes, which are essential processes in cellular signaling.
References
Abaterusso C, Gabaro G (2006) The role of glycosaminoglycans and sulodexide in treatment of diabetic nephropathy. Treat Endocrinol 5:211–212
Akutsu H, Seelig J (1981) Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry 20:7366–7373
Alexandrescu AT (2005) Amyloid accomplices and enforcers. Protein Sci 14:1–12
Ancsin JB (2003) Amyloidogenesis: historical and modern observations point to heparan sulfate proteoglycans as a major culprit. Amyloid 10:67–79
Anderson DR, Davis JL, Carraway KL (1977) Calcium-promoted changes of the human erythrocyte membrane. Involvement of spectrin, transglutaminase, and a membrane-bound protease. J Biol Chem 252:6617–6623
Bosman FT, Stamenkovic I (2003) Functional structure and composition of the extracellular matrix. J Pathol 200:423–428
Brzozowska I, Figaszewski ZA (2002) The equilibrium of phosphatidylcholine-cholesterol in moolayers at the air/water interface. Colloids Surf B 23:51–58
Bulow HE, Hobert O (2004) Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron 41:723–736
Cadena-Nava RD, Martin-Mirones JM, Vázquez-Martínez EA et al (2006) Direct observations of phase changes in Langmuir films of cholesterol. Rev Mex Fis 52:32–40
Camara CI, Wilke N (2017) Interaction of dectran derivatives with lipid monolayers and the consequential modulation of the film properties. Chem Phys Lipids 204:34–42
Capila I, Linhardt RJ (2002) Heparin-protein interactions. Angew Chem 41(30):390–412
Costin IS, Barnes GT (1975) Two-component monolayers. II. Surface pressure-area relations for the octadecanol-docosyl sulphate system. J Colloid Interface Sci 51:106–121
Davies JT, Rideal EK (1963) Interfacial phenomena. Academic, NewYork
Diederich A, Strobel M, Meier W, Winterhalter M (1999) Viscosity- and inertia-limited rupture of dextran-supported black lipid membranes. J Phys Chem B 103:1402–1407
Dynarowicz-Latka P, Kita K (1999) Molecular interaction in mixed monolayers at the air/water interface. Adv Colloid Interface Sci 79:1–17
Dynarowicz-Latka P, Seoane R, Miñones J Jr, Velo M (2002) Study of penetration of Amphotericin B into cholesterol or ergosterol containing dipalmitoyl phosphatidylcholine Langmuir monolayers. Colloids Surf B 27:249–263
Fisar Z (2005) Interactions between tricyclic antidepressants and phospholipid bilayer membranes. Gen Physiol Biophys 24:161–180
Fukamil K, Yamagishi S, Ueda S, Okuda S (2007) Novel therapeutic targets for diabetic nephropathy. Endocr Metab Immune Disord Drug Targets 7:83–92
Gaines GL (1966) Insoluble monolayers at liquid-gas interfaces. Interscience Publishers, New York
Hallak LK, Kwilas SA, Peeples ME (2007) Interaction between respiratory syncytial virus and glycosaminoglycans, including heparan sulfate. In: Sugrue RJ (ed) Glycovirology protocols. Humana Press, Totowa, pp 15–34
Horcas I, Fernández R, Gómez-Rodríguez JM, Colchero J, Gómez-Herrero J, Baro AM (2007) WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev Sci Instrum 78:013705–013708
Iannuzzi C, Irace G, Sirangelo I (2015) The effect of glycosaminoglycans (GAGs) on amyloid aggregation and toxicity. Molecules 20:2510–2528
Kim K, Choi SQ, Zell ZA, Squires TM, Zasadzinski JA (2013) Effect of cholesterol nanodomains on monolayer morphology and dynamics. Proc Natl Acad Sci USA 110:E3054–E3060
Laabs T, Carulli D, Geller HM, Fawcett JW (2005) Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol 15:116–120
Lindahl U, Li JP (2009) Interactions between heparan sulfate and proteins-design and functional implications. Int Rev Cell Mol Biol 276:105–159
Maget-Dana R (1999) The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim Biophys Acta 1462:109–140
Miñones J, Pais S, Miñones J et al (2009) Interactions between membrane sterols and phospholipids in model mammalian and fungi cellular membranes—a Langmuir monolayer study. Biophys Chem 140:69–77
Mislick KA, Baldeschwieler D (1996) Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc Natl Acad Sci USA 93:12349–12354
Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ (2005) Leuconostoc dextransucrase and dextran production, properties and applications. J Chem Technol Biotechnol 80:845–860
Nauck M, Rifai N (2000) Analytical performance and clinical efficacy of three routine procedures for LDL cholesterol measurement compared with the ultracentrifugation–dextran 21 sulfate-Mg2+ method. Clin Chim Acta 294:77–92
Nobre TM, Pavinatto FJ, Caseli L, Barros-Timmons A, Dynarowicz-Latka P, Oliveira ON (2015) Interactions of bioactive molecules and nanomaterials with Langmuir monolayers as cell membrane models. Thin Solid Films 593:158–188
Papy-Garcia D, Morin C, Huynh BM et al (2011) Glycosaminoglycans, protein aggregation and neurodegeneration. Curr Protein Pept Sci 12(3):258–268
Peetla C, Stine A, Labhasetwar V (2009) Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol Pharm 6:1264–1276
Ramalho-Santos J, Pedroso de Lima MC (2001) Fusion and infection of influenza and sendai viruses as modulated by dextran sulfate: a comparative study. Biosci Rep 21(3):293–304
Ross R, Steinem C, Galla H-J, Janshoff A (2001) Visualization of chemical and physical properties of calcium-induced domains in DPPC/DPPS Langmuir-Blodgett layers. Langmuir 17:2437–2445
Ruponen M, Honkakoski P, Tammi M, Urtti A (2004) Cell-surface glycosaminoglycans inhibit cation-mediated gene transfer. J Gene Med 6:405–414
Ruponen M, Ylä-Herttuala S, Urtti A (1999) Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: physicochemical and transfection studies. BBA 1415:331–341
Sabatini K, Mattila J-P, Kinnunen PKJ (2008) Interfacial behavior of cholesterol, ergosterol, and lanosterol in mixtures with DPPC and DMPC. Biophys J 95:2340–2355
Sahoo H, Schwille P (2013) Influence of glycosaminoglycans on lipid dynamics in supported phospholipid bilayers. Soft Matter 9:3859–3865
Santos HA, Chirea M, García-Morales V et al (2005a) Electrochemical study of interfacial composite nanostructures: polyelectrolyte/gold nanoparticle multilayers assembled on phospholipid/dextran sulfate monolayers at a liquid-liquid interface. J Phys Chem B 109:20105–20114
Santos HA, García-Morales V, Roozeman R-J et al (2005b) Interfacial interaction between dextran sulfate and lipid monolayers: an electrochemical study. Langmuir 21:5475–5484
Santos HA, García-Morales V, Murtomäki L et al (2007a) Preparation of nanostructures composed of dextran sulfate/ruthenium nanoparticles and their interaction with phospholipid monolayers at a liquid-liquid interface. J Electroanal Chem 599:194–202
Santos HA, Manzanares JA, Murtomäki L, Kontturi K (2007b) Thermodynamic analysis of binding between drugs and glycosaminoglycans by isothermal titration calorimetry and fluorescence spectroscopy. Eur J Pharm Sci 32:105–114
Saridaki T, Zampagni M, Mannini B et al (2012) Glycosaminoglycans (GAGs) suppress the toxicity of HypF-N prefibrillar aggregates. J Mol Biol 421:616–630
Simon SA, Lis LJ, Kauffman JW, MacDonald RC (1975) A calorimetric and monolayer investigation of the inluence of ions on the thermodynamic properties of phosphatidylcholine. Biochim Biophys Acta 375:317–326
Simons M, Krämer E-M, Macchi P et al (2002) Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease. J Cell Biol 157:327–336
Smits NC, Kurup S, Rops AL et al (2010) The heparan sulfate motif (GlcNS6S-IdoA2S)3, common in heparin, has a strict topography and is involved in cell behavior and disease. J Biol Chem 285:41143–41151
Stefaniu C, Brezesinski G, Möhwald H (2014) Langmuir monolayers as models to study processes at membrane surfaces. Adv Colloid Interface Sci 208:197–213
Uygun BE, Stojsih SE, Matthew HWT (2009) Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng Part A 15:3499–3512
Wiethoff CM, Smith JG, Koe GS, Middaugh CR (2001) The potential role of proteoglycans in cationic lipid-mediated gene delivery. Studies of the interaction of cationic lipid-DNA complexes with model glycosaminoglycans. J Biol Chem 276:32806–32813
Wight TN, Heinegård DK, Hascall VC (1991) Proteoglycans; structure and function. In: Hay ED (ed) Cell biology of extracellular matrix. Plenum Press, New York
Wydro P, Hąc-Wydro K (2007) Thermodynamic description of the interactions between lipids in ternary Langmuir monolayers: the study of cholesterol distribution in membranes. J Phys Chem B 111:2495–2502
Acknowledgements
The research was carried out with the equipment (Langmuir trough with BAM and Atomic Force Microscope) purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests regarding the publication of this article.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Makyła-Juzak, K., Chachaj-Brekiesz, A., Dynarowicz-Latka, P. et al. The Effect of Dextran Sulfate—as Model Glycosaminoglycan Analogue—on Membrane Lipids: DPPC, Cholesterol, and DPPC–Cholesterol Mixture. The Monolayer Study. J Membrane Biol 251, 641–651 (2018). https://doi.org/10.1007/s00232-018-0041-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-018-0041-z