Abstract
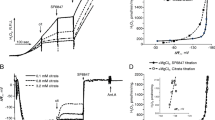
It is well known that the terpenoid ferutinin (4-oxy-6-(4-oxybenzoyloxy) dauc-8,9-en), isolated from the plant Ferula tenuisecta, considerably increases the permeability of artificial and cellular membranes to Ca2+-ions and produces apoptotic cell death in different cell lines in a mitochondria-dependent manner. The present study was designed for further evaluation of the mechanism(s) of mitochondrial effects of ferutinin using isolated rat liver mitochondria. Our findings provide evidence for ferutinin at concentrations of 5–27 µM to decrease state 3 respiration and the acceptor control ratio in the case of glutamate/malate as substrates. Ferutinin alone (10–60 µM) also dose-dependently dissipated membrane potential. In the presence of Ca2+-ions, ferutinin (10–60 µM) induced considerable depolarization of the inner mitochondrial membrane, which was partially inhibited by EGTA, and permeability transition pore formation, which was diminished partly by cyclosporin A, and did not influence markedly the effect of Ca2+ on mitochondrial respiration. Ruthenium Red, a specific inhibitor of mitochondrial calcium uniporter, completely inhibited Ca2+-induced mitochondria swelling and membrane depolarization, but did not affect markedly the stimulation of these Ca2+-dependent processes by ferutinin. We concluded that the mitochondrial effects of ferutinin might be primarily induced by stimulation of mitochondrial membrane Ca2+-permeability, but other mechanisms, such as driving of univalent cations, might be involved.








Similar content being viewed by others
Abbreviations
- ACR:
-
Acceptor control ratio
- CsA:
-
Cyclosporin A
- MCU:
-
Mitochondrial calcium uniporter
- MPT:
-
Mitochondrial permeability transition
- ROS:
-
Reactive oxygen species
- Ru red:
-
Ruthenium red
- FCCP:
-
Carbonyl cyanide p-trifluoro-methoxyphenyl hydrazine
References
Abramov AY, Duchen MR (2003) Action of ionomycin, 4-BrA23187 and novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium 33:101–112
Abramov AY, Zamaraeva MV, Hagelgans AI, Azimov RR, Krasilnikov OV (2001) Influence of plant terpenoids on the permeability of mitochondria and lipid bilayers. Biochim Biophys Acta 1512(1):98–110
Akerman KEO, Wikström MKF (1976) Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 6:191–197
Amin A, Gali-Muhtasib H, Osker M, Schneider-Stock R (2009) Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci 5(1):1–11
Arghiani N, Matin MM, Bahrami AR, Iranshashi M, Sazgarnia A, Rassouli FB (2014) Investigating anticancer properties of the sesquiterpene ferutinin on colon carcinoma cells, in vitro and in vivo. Life Sci 109:87–94
Azzolin L, Stockum S, Basso E, Petronilli V, Forte M, Bernardi P (2010) The mitochondrial permeability transition from yeast to mammals. FEBS Lett 584:2504–2509
Baranov SV, Stavrovskaya IG, Brown AM, Tyryshkin AM, Kristal BS (2008) Kinetic model for Ca2+-induced permeability transition in energized liver mitochondria discriminates between inhibitor mechanisms. J Biol Chem 283:665–676
Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476:341–345
Costantini P, Jacotot E, Decaudin D, Kroemer G (2000) Mitochondrion as a novel target of cancer chemotherapy. J Natl Cancer Inst 92(13):1042–1053
Colman-Saizarbitoria T, Boutros P, Amesty A, Bahsas A, Mathison Y, Garrido Mdel R, Israel A (2006) Ferutinin stimulates nitric oxide synthase activity in median eminence of the rat. J Ethnopharmacol 106:327–332
Dalla Via L, Garcia-Argaez AN, Martinez-Vazquez M, Grancara S, Martinis P, Toninello A (2014) Mitochondrial permeability transition as target of anticancer drugs. Curr Pharm Des 20:223–244
Dall’ Acqua S, Linardi MA, Maggi F, Nicoletti M, Petitto V, Innocenti G, Basso G, Viola G (2011) Natural daucane sesquiterpenes with antiproliferative activity against human tumor cells. Bioorg Med Chem 19:5876–5885
De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340
Dremza IK, Lapshina EA, Kujawa J, Zavodnik IB (2006) Oxygen-related processes in red blood cells exposed to tert-butyl hydroperoxide. Redox Rep 11:185–192
Dubis A, Zamaraeva MV, Siergiejczyk L, Charishnikova O, Shlyonsky V (2015) Ferutinin as a Ca(2+) complexone: lipid bilayers, conductometry, FT-IR, NMR studies and DFT-B3LYP calculations. Dalton Trans 44(37):16506–16515
Duchen MR (2004) Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med 25(4):365–451
Ferretti M, Bertoni L, Cavani F, Benincasa M, Sena P, Carnevale G, Zavatti M, Viesti VD, Zanoli P, Palumbo C (2012) Structural and histomorphometric evaluations of ferutinin effects on the uterus of ovariectomized rats during osteoporosis treatment. Life Sci 90(3–4):161–168
Ferretti M, Cavani F, Manni P, Carnevale G, Bertoni L, Zavatti M, Palumbo C (2014) Ferutinin dose-dependent effects on uterus and mammary gland in ovariectomized rats. Histol Histopathol 29(8):1027–1037
Fulda S (2010) Modulation of apoptosis by natural products for cancer therapy. Planta Med 76(11):1075–1079
Gao M, Wong SY, Lau PM, Kong SK (2013) Ferutinin induces in vitro eryptosis/erythroptosis in human erythrocytes through membrane permeabilization and calcium influx. Chem Res Toxicol 26(8):1218–1228
Geroushi A, Auzi AA, Elhwuegi AS, Elzawam F, Berretu A, Hanar L, Sarker SD (2011) Antiinflammatory sesquiterpenes from the root oil of Ferula hermonis. Phytother Res 25:774–777
Golovach NG, Cheshchevik VT, Lapshina EA, Ilyich TB, Zavodnik IB (2017) Calcium-induced mitochondrial permeability transitions: parameters of Ca2+ ion interactions with mitochondria and effects of oxidative agents. J Memb Biol 250:225–236
Gorlach S, Fichna J, Lewandowska U (2015) Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett 366:141–149
Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW, Koch WJ, Cheung JY, Madesh M (2014) SLC25A23 augments mitochondrial Ca2+ uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell 25:936–947
Ignatkov V, Akhmedkhodzhaeva KS, Babichev VN (1990) The effect of tefesterol on the secretion of luteinizing hormone from the hypophysis. Farmakol Toksikol 53:37–38
Ikeda K, Arao Y, Otsuka H, Nomoto S, Horiguchi H, Kato S, Kayama F (2002) Terpenoids found in the umbelliferae family act as agonists/antagonists for ER(alpha) and ERbeta: differential transcription activity between ferutinine-liganded ER(alpha) and ERbeta. Biochem Biophys Res Commun 291:354–360
Johnson D, Lardy HA (1967) Isolation of liver or kidney mitochondria. Methods Enzymol 10:94–101
Jonas EA, Porter CA Jr, Beutner G, Mnatsakanyan N, Alavian K (2015) Cell death disguised: the mitochondrial permeability transition pore as the c-subunit of the F1FO ATP synthase. Pharmacol Res 99:382–392
Kirichok Y, Krapivinsky G, Clapham DE (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427:360–364
Korotkov SM, Konovalova SA, Brailovskaya IV, Saris NE (2016) To involvement the conformation of the adenine nucleotide translocase in opening the Tl(+)-induced permeability transition pore in Ca(2+)-loaded rat liver mitochondria. Toxicol In Vitro 32:320–332. https://doi.org/10.1016/j.tiv.2016.01.015
Korotkov SM, Saris NE (2011) Influence of Tl(+) on mitochondrial permeability transition pore in Ca(2+)-loaded rat liver mitochondria. J Bioenerg Biomembr 43(2):149–162. https://doi.org/10.1007/s10863-011-9341-z
Kowaltowski AJ (2000) Alternative mitochondrial functions in cell physiopathology: beyond ATP production. Braz J Med Biol Res 33(2):241–250
Lhuillier A, Fabre N, Cheble E, Oueida F, Maurel S, Valentin A, Fouraste I, Moulis C (2005) Daucane sesquiterpenes from Ferula hermonis. J Nat Prod 68:468–471
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Macho A, Blanco-Molina M, Spagliardi P, Appendino G, Bremner P, Heinrich M, Fiebich BL, Muñoz E (2004) Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem Pharmacol 68(5):875–883
Matin MM, Nakhaeizadeh H, Bahrami AR, Iranshahi M, Arghiani N, Rassouli FB (2014) Ferutinin, an apoptosis inducing terpenoid from Ferula ovina. Asian Pac J Cancer Prev 15:2123–2128
Modzelewska A, Sur S, Kumar SK, Khan SR (2005) Sesquiterpenes: natural products that decrease cancer growth. Curr Med Chem Anticancer Agents 5(5):477–499
Mondal S, Bandyopadhyay S, Ghosh MK, Mukhoppadhyay S, Roy S, Mandal C (2012) Natural products: promising resources for cancer drug discovery. Anticancer Agents Med Chem 12(1):49–75
Moore AL, Bonner WD (1982) Measurements of membrane potentials in plant mitochondria with the safranine method. Plant Physiol 70:1271–1276
Ouyang L, Luo Y, Tian M, Zhang SY, Lu R, Wang JH, Kasimu R, Li X (2014) Plant natural products: from traditional compounds to new emerging drugs in cancer therapy. Cell Prolif 47(6):506–515
Pandya JD, Nukala VN, Sullivan PG (2013) Concentration dependent effect of calcium on brain mitochondrial bioenergetics and oxidative stress parameters. Front Neuroenergetics 5:10. https://doi.org/10.3389/fnene.2013.00010
Petronilli V, Cola C, Bernardi P (1993a) Modulation of the mitochondrial cyclosporin A—sensitive permeability transition pore. II. The minimal requirements for pore induction underscore a key role for transmembrane electrical potential, matrix pH, and matrix Ca2+. J Biol Chem 268:1011–1016
Petronilli V, Cola C, Massari S, Colonna R, Bernardi P (1993b) Physiological effectors modify voltage sensing by the cyclosporine A—sensitive permeability transition pore of mitochondria. J Biol Chem 268:21939–21945
Poli F, Appendino G, Sacchetti G, Ballero M, Maggiano N, Ranelletti DO (2005) Antiproliferative effects of daucane esters from Ferula communis and F. arrigonii on human colon cancer cell lines. Phytother Res 19:152–157
Safi R, Rodriguez F, Hilal G, Diab-Assaf M, Diab Y, El-Sabban M, Najjar F, Delfourne E (2016) Hemisynthesis, antitumoral effect, and molecular docking studies of ferutinin and its analogues. Chem Biol Drug Des 87(3):382–397
Saidkhodzjaev AJ, Nikonov GK (1973) The constituents of ferutinin. Chem Nat Prod 1:28–30
Suta S, Maggi F, Nicoletti M, Baldan V, Dall Acqua S (2017) New drugs from old natural compounds: scarcely investigated sesquiterpenes as new possible therapeutic agents. Curr Med Chem. https://doi.org/10.2174/0929867324666170404150351
Szewczyk A, Wojtczak L (2002) Mitochondria as a pharmacological target. Pharmacol Rev 54:101–127
Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins W (2004) Mitochondrial localization of estrogen receptor β. Proc Natl Acad Sci USA 101(12):4130–4135
Zamaraeva MV, Hagelgans AI, Abramov AY, Ternovsky VI, Merzlyak PG, Tashmukhamedov BA, Saidkhodzjaev AI (1997) Ionophoretic properties of ferutinin. Cell Calcium 22(4):235–241
Zamaraeva MV, Hagelgans AI, Lubnina AI, Abramov AY, Ahmedhodjaeva HS, Saidhodjaev AI, Glazyrina NG (1999) Hormonal activity and membrane action of plants terpenoids. Cell Mol Biol Lett 4(2):189
Zamaraeva M, Charishnikova O, Saidkhodjaev A, Isidorov V, Granosik M, Rozalski M, Watala C (2010) Calcium mobilization by the plant estrogen ferutinin does not induce blood platelet aggregation. Pharmcol Rep 62:1117–1126
Zavatti M, Bertoni L, Maraldi T, Resca E, Berreti F, Guida M, La Sala GB, De Pol A (2015) Critical-size bone defect repair using amniotic fluid stem cell/collagen constructs: effect of oral ferutinin treatment in rats. Life Sci 121:174–183
Zavodnik IB, Dremza IK, Cheshchevik VT, Lapshina EA, Zamaraewa M (2013) Oxidative damage of rat liver mitochondria during exposure to t-butyl hydroperoxide. Role of Ca2+ ions in oxidative processes. Life Sci 92(23):1110–1117
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
The informed consent was obtained from all the individual participants involved in the study.
Rights and permissions
About this article
Cite this article
Ilyich, T., Charishnikova, O., Sekowski, S. et al. Ferutinin Induces Membrane Depolarization, Permeability Transition Pore Formation, and Respiration Uncoupling in Isolated Rat Liver Mitochondria by Stimulation of Ca2+-Permeability. J Membrane Biol 251, 563–572 (2018). https://doi.org/10.1007/s00232-018-0032-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-018-0032-0