Abstract
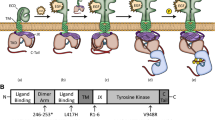
Fibroblast growth factor receptor 3 (FGFR3) is a single-pass membrane protein and a member of the receptor tyrosine kinase family of proteins that is involved in the regulation of skeletal growth and development. FGFR3 has three distinct domains: the ligand binding extracellular domain, the cytosolic kinase domain and the transmembrane domain (TMD). Previous work with the isolated FGFR3 TMD has shown that it has the ability to dimerize. Clinical and genetic studies have also correlated mutations in the TMD with a variety of skeletal and cranial dysplasias and cancer. Although the structures of the extracellular and cytosolic domains of FGFR3 have been solved, the structure of the TMD dimer is still unknown. Furthermore, very little is known regarding the effects of pathogenic mutations on the TMD dimer structure. We, therefore, carried out ToxR activity assays to determine the role of the SmXXXSm motif in the dimerization of the FGFR3 TMD. This motif has been shown to drive the association of many transmembrane proteins. Our results indicate that the interaction between wild-type FGFR3 TMDs is not mediated by two adjacent SmXXXSm motifs. In contrast, studies using the TMD carrying the pathogenic A391E mutation suggest that the motifs play a role in the dimerization of the mutant TMD. Based on these observations, here we report a new mechanistic model in which the pathogenic A391E mutation induces a structural change that leads to the formation of a more stable dimer.




Similar content being viewed by others
References
Bocharov EV, Mayzel ML, Volynsky PE, Mineev KS, Tkach EN, Ermolyuk YS, Schulga AA, Efremov RG, Arseniev AS (2010) Left-handed dimer of EphA2 transmembrane domain: helix packing diversity among receptor tyrosine kinases. Biophys J 98:881–889
Brosig B, Langosch D (1998) The dimerization motif of the glycophorin A transmembrane segment in membranes: importance of glycine residues. Protein Sci 7:1052–1056
Call ME, Wucherpfennig KW, Chou JJ (2010) The structural basis for intramembrane assembly of an activating immunoreceptor complex. Nat Immunol 11:1023–1029
Chen F, Degnin C, Laederich M, Horton WA, Hristova K (2011) The A391E mutation enhances FGFR3 activation in the absence of ligand. Biochim Biophys Acta 1808:2045–2050
Cymer F, Veerappan A, Schneider D (2012) Transmembrane helix–helix interactions are modulated by the sequence context and by lipid bilayer properties. Biochim Biophys Acta 1818:963–973
Doura AK, Kobus FJ, Dubrovsky L, Hibbard E, Fleming KG (2004) Sequence context modulates the stability of a GxxxG-mediated transmembrane helix–helix dimer. J Mol Biol 341:991–998
Finger C, Escher C, and Schneider D (2009) The single transmembrane domains of human receptor tyrosine kinases encode self-interactions. Sci Signal 2:ra56
He L, Hristova K (2008) Pathogenic activation of receptor tyrosine kinases in mammalian membranes. J Mol Biol 384:1130–1142
He L, Hoffmann AR, Serrano C, Hristova K, Wimley WC (2011) High-throughput selection of transmembrane sequences that enhance receptor tyrosine kinase activation. J Mol Biol 412:43–54
He L, Serrano C, Niphadkar N, Shobnam N, Hristova K (2012) Effect of the G375C and G346E achondroplasia mutations on FGFR3 activation. PLoS ONE 7:e34808
Herrmann JR, Fuchs A, Panitz JC, Eckert T, Unterreitmeier S, Frishman D, Langosch D (2010) Ionic interactions promote transmembrane helix–helix association depending on sequence context. J Mol Biol 396:452–461
Langosch D, Brosig B, Kolmar H, Fritz HJ (1996) Dimerisation of the glycophorin a transmembrane segment in membranes probed with the ToxR transcription activator. J Mol Biol 263:525–530
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134
Lemmon MA, Treutlein HR, Adams PD, Brünger AT, Engelman DM (1994) A dimerization motif for transmembrane alpha-helices. Nat Struct Biol 1:157–163
Li E, Hristova K (2006) Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry 45:6241–6251
Li E, You M, Hristova K (2005) SDS-PAGE and FRET suggest weak interactions between FGFR3 TM domains in the absence of extracellular domains and ligands. Biochemistry 44:352–360
Li E, You M, Hristova K (2006) FGFR3 dimer stabilization due to a single amino acid pathogenic mutation. J Mol Biol 356:600–612
Li E, Wimley WC, Hristova K (2012) Transmembrane helix dimerization: beyond the search for sequence motifs. Biochim Biophys Acta 1818:183–193
MacKenzie KR, Prestegard JH, Engelman DM (1997) A transmembrane helix dimer: structure and implications. Science 276:131–133
Melnyk RA, Kim S, Curran AR, Engelman DM, Bowie JU, Deber CM (2004) The affinity of GXXXG motifs in transmembrane helix–helix interactions is modulated by long-range communication. J Biol Chem 279:16591–16597
Merzlyakov M, Chen L, Hristova K (2007) Studies of receptor tyrosine kinase transmembrane domain interactions: the EmEx-FRET method. J Membr Biol 215:93–103
Mineev KS, Bocharov EV, Pustovalova YE, Bocharova OV, Chupin VV, Arseniev AS (2010) Spatial structure of the transmembrane domain heterodimer of ErbB1 and ErbB2 receptor tyrosine kinases. J Mol Biol 400:231–243
Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B (2006) A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J Biol Chem 281:22744–22751
Oates J, King G, Dixon AM (2010) Strong oligomerization behavior of PDGFbeta receptor transmembrane domain and its regulation by the juxtamembrane regions. Biochim Biophys Acta 1798:605–615
Schneider D, Engelman DM (2004) Motifs of two small residues can assist but are not sufficient to mediate transmembrane helix interactions. J Mol Biol 343:799–804
Sulistijo ES, Jaszewski TM, MacKenzie KR (2003) Sequence-specific dimerization of the transmembrane domain of the “BH3-only” protein BNIP3 in membranes and detergent. J Biol Chem 278:51950–51956
van Rhijin B, van Tilborg A, Lurkin I, Bonaventure J, de Vries A, Thiery JP, van der Kwast TH, Zwarthoff E, Radvanyi F (2002) Novel fibroblast growth factor receptor 3 (FGFR3) mutations in bladder cancer previously identified in non-lethal skeletal disorders. Eur J Hum Genet 10:819–824
You M, Li E, Wimley WC, Hristova K (2005) FRET in liposomes: measurements of TM helix dimerization in the native bilayer environment. Anal Biochem 340:154–164
You M, Li E, Hristova K (2006) The achondroplasia mutation does not alter the dimerization energetics of FGFR3 transmembrane domain. Biochemistry 45:5551–5556
You M, Spangler J, Li E, Han X, Ghosh P, Hristova K (2007) Effect of pathogenic cysteine mutations on FGFR3 transmembrane domain dimerization in detergents and lipid bilayers. Biochemistry 46:11039–11046
Acknowledgments
We thank Dr. Dieter Langosch at the Technical University of Munich for the ToxR vector plasmids and the FHK12 and PD28 E. coli cells. We are also grateful to the Alexander von Humboldt Foundation and the Saint Joseph’s University chapter of Sigma Xi, the Research Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mudumbi, K.C., Julius, A., Herrmann, J. et al. The Pathogenic A391E Mutation in FGFR3 Induces a Structural Change in the Transmembrane Domain Dimer. J Membrane Biol 246, 487–493 (2013). https://doi.org/10.1007/s00232-013-9563-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-013-9563-6