Abstract
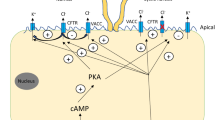
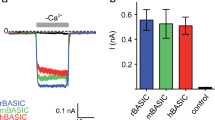
Volume-activated chloride channel (VACC) plays vital roles in many physiological functions. In bile duct epithelium, VACC actively participates in biliary secretion and cell volume regulation, and it mediates regulatory volume decrease (RVD). Recently, we have shown that mouse cholangiocytes have an intact RVD via VACC and K+ conductance. However, such cell volume regulation was not studied in the normal human cholangiocyte. Volume measurement by Coulter counter and whole-cell patch clamp technique were used to characterize the RVD and VACC in human cholangiocyte cell line (HBDC). When exposed to hypotonic solution, HBDC exhibited an intact RVD, which was inhibited by 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), NPPB (5-nitro-2′- (3-phenylpropylamino)-benzoate), DIDS (4,4′-diisothiocyanatostilbene-2-disulfonic acid), and tamoxifen, but was not affected by the removal of extracellular calcium. During RVD, HBDC exhibited large, outwardly rectifying currents and time-dependent inactivation at positive potential. The amplitude of the outward current was approximately 3 times of that of the inward current, and this volume-activated current returned to the baseline when switched to isotonic solution. The amplitude and reversal potential of the volume-activated current was dependent on Cl− concentration, and the VACC was significantly inhibited by replacing chloride with gluconate, glutamate, sucrose, and acetate in the hypotonic solution. In addition, classical VACC inhibitors, such as NPPB or tamoxifen, inhibited the VACC. These inhibitory effects were reversible with washing out the inhibitors from the bath solution. The present study is the first to characterize and show that HBDC has an intact RVD, mediated by VACC, which has similar electrophysiological characteristics as that in mouse cholangiocytes.




Similar content being viewed by others
References
Banderali U, Roy G (1992) Activation of K+ and Cl− channels in MDCK cells during volume regulation in hypotonic media. J Membr Biol 126:219–234
Bhaskar KR, Turner BS, Grubman SA, Jefferson DM, LaMont JT (1998) Dysregulation of proteoglycan production by intrahepatic biliary epithelial cells bearing defective (Delta-f508) cystic fibrosis transmembrane conductance regulator. Hepatology 27:7–14
Chen BY, Nicol G, Cho WK (2004) Electrophysiological characterization of volume activated chloride currents in mouse cholangiocyte cell line. Am J Physiol Gastrointest Liver Physiol 287:1158–1167
Chen BY, Nicol G, Cho WK (2007) Role of calcium on volume activated chloride current in mouse cholangiocyte. J Membr Biol 215:1–13
Cho WK (2002) Characterization of regulatory volume decrease in freshly isolated mouse cholangiocytes. Am J Physiol Gastrointest Liver Physiol 283:G1320–G1327
Cho WK, Siegrist VJ, Zinzow W (2004) Impaired regulatory volume decrease (RVD) in freshly isolated cholangiocytes from cystic fibrosis mice. J Biol Chem 279:14610–14618
Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA (1993) Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest 91:319–328
Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM (1994) Regulation of pHi by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol Gastrointest Liver Physiol 266:G1060–G1070
Grubman SA, Fang SL, Mulberg AE, Perrone RD, Rogers LC, Lee DW, Armentano D et al (1995) Correction of the cystic fibrosis defect by gene complementation in intrahepatic biliary epithelial cell lines derived from patients with cystic fibrosis. Gastroenterology 108:584–592
Hazama A, Okada Y (1988) Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol 402:687–702
Hoffmann EK, Simonsen LO (1989) Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev 69:315–382
Kirk K (1997) Swelling-activated organic osmolyte channels. J Membr Biol 158:1–16
Lewis RS, Ross PE, Cahalan MD (1993) Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol 101:801–826
Li GR, Yang BF, Sun HY, Baumgarten CM (2000) Existence of a transient outward K current in guinea pig cardiac myocytes. Am J Physiol Heart Circ Physiol 279:H130–H138
Mennone A, Alvaro D, Cho W, Boyer JL (1995) Isolation of small polarized bile duct units. Proc Natl Acad Sci USA 92:6527–6531
Mignen O, Le Gall C, Harvey BJ, Thomas S (1999) Volume regulation following hypotonic shock in isolated crypts of mouse distal colon. J Physiol 515:501–510
Mintenig GM, Valverde MA, Sepulveda FV, Gill DR, Hyde SC, Kirk J, Higgins CF (1993) Specific inhibitors distinguish the chloride channel and drug transporter functions associated with the human multidrug resistance P-glycoprotein. Recept Channels 1:305–313
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Park JS, Choi YJ, Vicki J. Siegrist VJ, Ko YS, Won Kyoo Cho WK (2007) Permissive role of calcium on regulatory volume decrease in freshly isolated mouse cholangiocytes. Pflugers Arch Eur J Physiol 455:261–271
Roman RM, Wang Y, Fitz JG (1996) Regulation of cell volume in a human biliary cell line: activation of K+ and Cl− currents. Am J Physiol 271:G239–G248
Schmid A, Blum R, Krause E (1998) Characterization of cell volume–sensitive chloride currents in freshly prepared and cultured pancreatic acinar cells from early postnatal rats. J Physiol 513(pt 2):453–465
Acknowledgments
W.C. was supported by KO8 DK02613, R03 DK61409, Cystic Fibrosis Research Grant, Indiana University Biomedical Research Grant, and Indiana University Research Support Fund. We appreciate technical help of Guangqin Zhang in maintaining cell cultures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, B., Jefferson, D.M. & Cho, W.K. Characterization of Volume-Activated Chloride Currents in Regulatory Volume Decrease of Human Cholangiocyte. J Membrane Biol 235, 17–26 (2010). https://doi.org/10.1007/s00232-010-9252-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9252-7