Abstract
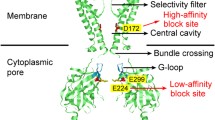
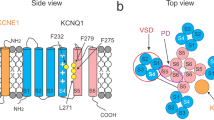
An E224G mutation of the Kir2.1 channel generates intrinsic inward rectification and single-channel fluctuations in the absence of intracellular blockers. In this study, we showed that positively charged residues H226, R228 and R260, near site 224, regulated the intrinsic inward rectification and single-channel properties of the E224G mutant. By carrying out systematic mutations, we found that the charge effect on the intrinsic inward rectification and single-channel conductance is consistent with a long-range electrostatic mechanism. A Kir1.1 channel where the site equivalent to E224 in the Kir2.1 channel is a glycine residue does not show inward rectification or single-channel fluctuations. The G223K and N259R mutations of the Kir1.1 channel induced intrinsic inward rectification and reduced the single-channel conductance but did not generate large open-channel fluctuations. Substituting the cytoplasmic pore of the E224G mutant into the Kir1.1 channel induced open-channel fluctuations and intrinsic inward rectification. The single-channel conductance of the E224G mutant showed inward rectification. Also, a voltage-dependent gating mechanism decreased open probability during depolarization and contributed to the intrinsic inward rectification in the E224G mutant. In addition to an electrostatic effect, a close interaction of K+ with channel pore may be required for generating open-channel fluctuations in the E224G mutant.









Similar content being viewed by others
References
Alagem N, Dvir M, Reuveny E (2001) Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol 534:381–393
Brelidze TI, Niu X, Magleby KL (2003) A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc Natl Acad Sci USA 100:9017–9022
Chandler WK, Hodgkin AL, Meves H (1965) The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol 180:821–836
Chang HK, Yeh SH, Shieh RC (2005) A ring of negative charges in the intracellular vestibule of Kir2.1 channel modulates K+ permeation. Biophys J 88:243–254
Choe H, Sackin H, Palmer LG (2000) Permeation properties of inward-rectifier potassium channels and their molecular determinants. J Gen Physiol 115:391–404
Dani JA (1986) Ion-channel entrances influence permeation. Net charge, size, shape, and binding considerations Biophys J 49:607–618
Doyle AD, Morais CJ, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280:69–77
Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM (1994) Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science 266:1068–1072
Fujiwara Y, Kubo Y (2006) Functional roles of charged amino acid residues on the wall of the cytoplasmic pore of Kir2.1. J Gen Physiol 127:401–419
Green WN, Andersen OS (1991) Surface charges and ion channel function. Annu Rev Physiol 53:341–359
Green WN, Weiss LB, Andersen OS (1987) Batrachotoxin-modified sodium channels in planar lipid bilayers. Ion permeation and block. J Gen Physiol 89:841–872
Guo D, Lu Z (2002) IRK1 inward rectifier K+ channels exhibit no intrinsic rectification. J Gen Physiol 120:539–551
Hamill OP, Marty A, Heher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch 391:85–100
Hilgemann DW (1995) The giant membrane patch. In: Sakmann B, Neher E (eds), Single-Channel Recording. New York: Plenum Press, pp 307–328
Hille B (2001) Potassium channels and chloride channels. In: Ionic Channels of Excitable Membranes. Sinauer, MA: Sunderland, pp 115–139
Hille B, Woodhull AM, Shapiro BI (1975) Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci 270:301–318
Ho K, CG Nichols, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC (1993) Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362:31–38
Imoto K, Busch C, Sakmann B, Mishina M, Konno T, Nakai J, Bujo H, Mori Y, Fukuda K, Numa S (1988) Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature 335:645–648
Ishihara K, Mitsuiye T, Noma A, Takano M (1989) The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J Physiol 419:297–320
Kell MJ, DeFelice LJ (1988) Surface charge near the cardiac inward-rectifier channel measured from single-channel conductance. J Membr Biol 102:1–10
Kienker P, Tomaselli G, Jurman M, Yellen G (1994) Conductance mutations of the nicotinic acetylcholine receptor do not act by a simple electrostatic mechanism. Biophys J 66:325–334
Kubo Y, Baldwin T, Jan Y, Jan L (1993) Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362:127–133
Kubo Y, Murata Y (2001) Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K+ channel. J Physiol 531:645–660
Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA (2003) Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300:1922–1926
Lin CW, Chen TY (2003) Probing the pore of ClC-0 by substituted cysteine accessibility method using methane thiosulfonate reagents. J Gen Physiol 122:147–159
Lopatin AN, Makhina EN, Nichols CG (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372:366–369
Lu Z, MacKinnon R (1994) Electrostatic tuning of Mg2+ affinity in an inward- rectifier K+ channel. Nature 371:243–246
MacKinnon R, Latorre R, Miller C (1989) Role of surface electrostatics in the operation of a high- conductance Ca2+-activated K+ channel. Biochemistry 28:8092–8099
Matsuda H, Oishi K, Omori K (2003) Voltage-dependent gating and block by internal spermine of the murine inwardly rectifying K+ channel, Kir2.1. J Physiol 548:361–371
Matsuda H, Saigusa A, Irisawa H (1987) Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature 325:156–159
Meltzer RH, Lurtz MM, Wensel TG, Pedersen SE (2006) Nicotinic acetylcholine receptor channel electrostatics determined by diffusion-enhanced luminescence energy transfer. Biophys J 91:1315–1324
Nimigean CM, Chappie JS, Miller C (2003) Electrostatic tuning of ion conductance in potassium channels. Biochemistry 42:9263–9268
Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S (2005) Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci 8:279–287
Shieh RC, Chang JC, Arreola J (1998) Interaction of Ba2+ with the pores of the cloned inward rectifier K+ channels Kir2.1 expressed in Xenopus oocytes. Biophys J 75:2313–2322
Shieh RC, John SA, Lee JK, Weiss JN (1996) Inward rectification of the IRK1 channel expressed in Xenopus oocytes: effects of intracellular pH reveal an intrinsic gating mechanism. J Physiol 494(Pt 2):363–376
Shioya T, Matsuda H, Noma A (1993) Fast and slow blockades of the inward-rectifier K+ channel by external divalent cations in guinea-pig cardiac myocytes. Pfluegers Arch 422:427–435
So I, Ashmole I, Hoh H, Park C, Spencer P, Leyland M, Stanfield P (2003) Intrinsic gating in inward rectifier potassium channels (Kir2.1) with low polyamine affinity generated by site directed mutagenesis. Korean J Physiol Pharmacol 7:131–142
Stauffer DA, Karlin A (1994) Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry 33:6840–6849
Taglialatela M, Wible BA, Caporaso R, Brown AM (1994) Specification of pore properties by the carboxyl terminus of inwardly rectifying K+ channels. Science 264:844–847
Vandenberg C (1987) Inward rectification of a potassium channel in cardiac ventricular cells depends of internal magnesium ions. Proc Natl Acad Sci USA 84:2560–2564
Xie LH, John SA, Ribalet B, Weiss JN (2004) Regulation of gating by negative charges in the cytoplasmic pore in the Kir2.1 channel. J Physiol 561:159–168
Xie LH, John SA, Weiss JN (2002) Spermine block of the strong inward rectifier potassium channel Kir2.1: dual roles of surface charge screening and pore block. J Gen Physiol 120:53–66
Yang J, Jan YN, Jan LY (1995) Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron 14:1047–1054
Yeh SH, Chang HK, Shieh RC (2005) Electrostatics in the cytoplasmic pore produce intrinsic inward rectification in Kir2.1 channels. J Gen Physiol 126:551–562
Zhang YY, Robertson JL, Gray DA, Palmer LG (2004) Carboxy-terminal determinants of conductance in inward-rectifier K channels. J Gen Physiol 124:729–739
Acknowledgement
We thank Drs. Lily Jan and James N. Weiss for kindly providing the Kir2.1 and Kir1.1 clones, respectively. We are grateful to Dr. Tom Barkas for reading and editing the manuscript. This work was supported by the Academia Sinica and by the National Science Council of Taiwan (grants 94–2320-B-001–025 and 95–2320-B001–011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, HK., Yeh, SH. & Shieh, RC. Charges in the Cytoplasmic Pore Control Intrinsic Inward Rectification and Single-Channel Properties in Kir1.1 and Kir2.1 Channels. J Membrane Biol 215, 181–193 (2007). https://doi.org/10.1007/s00232-007-9017-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-007-9017-0