Abstract
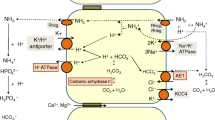
Transition from antidiuresis to diuresis exposes cortical collecting duct cells (CCD) to asymmetrical changes in environment osmolality, inducing an osmotic stress, which activates numerous membrane-associated events. The aim of the present work was to investigate, either in the presence or not of AQP2, the transepithelial osmotic water permeability (Posm) following cell exposure to asymmetrical hyper- or hypotonic gradients. For this purpose, transepithelial net volume fluxes were recorded every minute in two CCD cell lines: one not expressing AQPs (WT-RCCD1) and another stably transfected with AQP2 (AQP2-RCCD1). Our results demonstrated that the rate of osmosis produced by a given hypotonic shock depends on the gradient direction (osmotic rectification) only in the presence of apical AQP2. In contrast, hypertonic shocks elicit Posm rectification independently of AQP2 expression, and this phenomenon may be linked to modulation of basolateral membrane permeability. No asymmetry in transepithelial resistance was observed under hypo- or hypertonicity, indicating that rectification cannot be attributed to a shunt through the tight junction path. We conclude that osmotic rectification may be explained in terms of dynamical changes in membrane permeability probably due to activation/incorporation of AQPs or transporters to the plasma membrane via some mechanism triggered by osmolality.





Similar content being viewed by others
References
Agre P., Kozono D. 2003. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 555:71–78
Blot-Chabaud M., Laplace M., Cluzeaud F., Capurro C., Cassingena C., Vandewalle A., Farman N., Bonvalet J.P. 1996. Characteristics of a rat cortical collecting duct cell line that maintains high transepithelial resistance. Kidney Intern. 50:367–376
Brown D. 2003. The ins and outs of aquaporin-2 trafficking. Am. J. Physiolo. 284:F893–F901
Burg M., Helman S., Grantham J., Orloff J. 1969. Effect of vasopressin on the permeability of isolated rabbit cortical collecting tubules to urea, acetamide and thiourea. Excerpta. Med. Sect II 20:163–169
Candia O.A., Mia A., Yorio T. 1997. Evidence of basolateral water permeability regulation in amphibian urinary bladder. Biol. Cell. 89:331–339
Candia O.A., Patarca R., Alvarez L.J. 1998. Reduction of water permeability by anisotonic solutions in frog corneal epithelium. Invest. Ophthalmol. Vis. Sci. 39:378–384
Capurro C., Rivarola V., Kierbel A., Farman N., Blot-Chabaud M., Parisi M. 2001. Vasopressin regulates water flow in a rat cortical collecting duct cell line not containing known aquaporins. J. Membrane Biol. 149:63–70
Dainty J. 1963. The polar permeability of plant cell membranes to water. Protoplasma 57:220–228
Deen P.M., Verdijk M.A., Knoers N.V., Wieringa B., Monnens L.A., van Os C.H., van Oost B. 1994. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 264:92–95
DiBona D.R. 1983. Cytoplasmic involvement in ADH-mediated osmosis across toad urinary bladder. Am. J. Physiol. 245:C297–C307
Djelidi S., Fay M., Cluzeaud F., Escoubet B., Eugene E., Capurro C., Bonvalet J.P., Farman N., Blot-Chabaud M. 1997. Transcriptional regulation of sodium transport by vasopressin in renal cells. J. Biol. Chem. 272:32918–32924
Djelidi S., Fay M., Cluzeaud F., Thomas-Soumarmon A., Bonvalet J.P., Farman N., Blot-Chabaud M. 1999. Vasopressin stimulates long-term net chloride secretion in cortical collecting duct cells. FEBS. Lett. 400:533–538
Dorr R.A., Kierbel A., Vera J., Parisi M. 1997. A new data-acquisition system for the measurement of the net volume flux across epithelia. Computer Methods and Programs in Biomedicine 53:9–14
Finkelstein A. 1987. Water movement through lipid bilayers, pores and plasma membranes. John Wiley & Sons, USA
Fischbarg J. 1997. Mechanism of fluid transport across corneal endothelium and other epithelial layers: a possible explanation based on cyclic cell volume regulatory changes. Br. J. Ophthalmol. 81:85–89
Fischbarg J., Warshavsky C.R., Lim J.J. 1977. Pathways for hydraulically and osmotically-induced water flows across epithelia. Nature 266:71–74
Ford, P., Rivarola, V., Chara, O., Blot-Chabaud, M., Cluzeaud, F., Farman, N., Parisi, M., Capurro, C. 2005. Volume regulation in cortical collecting duct cells: role of AQP2. Biol. Cell. Epubdoi:10.1042/BC20040116
Ford P., Rivarola V., Kierbel A., Chara O., Blot-Chabaud M., Farman N., Parisi M., Capurro C. 2002. Differential role of Na+/H+ exchange isoforms NHE-1 and NHE-2 in a rat cortical collecting duct cell line. J. Membrane. Biol. 190:117–125
Gratham J.J., Orloff J. 1968. Effect of prostaglandin E 1 on the permeability response of the isolated collecting tubule to vasopresin, adenosine 3′,5′-monophosphate and theophyline. J. Clin. Invest. 47:1154–1161
Harris H.W. Jr., Botelho B. Zeidel M.L., Strange K. 1992. Cytoplasmic dilution induces antidiuretic hormone water channel retrieval in toad urinary bladder. Am. J. Physiol. 263:F163–F170
Kedem O., Katchalsky A. 1963. Permeability of composite membranes. Part III. Series array of elements. Trans. Faraday Soc. 59:1941–1953
Kirk D.L., DiBona D.R., Schafer J.A. 1984. Morphologic response of the rabbit cortical collecting tubule to peritubular hypotonicity: quantitative examination with differential interference contrast microscopy. J. Membrane Biol. 79:53–64
Kirk D.L., Schafer J.A., DiBona D.R. 1984. Quantitative analysis of the structural events associated with antidiuretic hormone-induced volume reabsorption in the rabbit cortical collecting tubule. J. Membrane Biol. 79:65–74
Kuwahara M., Shi L.B., Marumo F., Verkman A.S. 1991. Transcellular water flow modulates water channel exocytosis and endocytosis in kidney collecting tubule. J. Clin. Invest. 88:423–429
Loeschke K., Bentzel C.J., Csaky T.Z. 1970. Asymmetry of osmotic flow in frog intestine: functional and structural correlation. Am. J. Physiol. 218:1723–1731
Maric K., Wiesner B., Lorenz D., Klussmann E., Betz T., Rosenthal W. 2001. Cell volume kinetics of adherent epithelial cells measured by laser scanning reflection microscopy: determination of water permeability changes of renal principal cells. Biophys. J. 80:1783–1790
Moshelion M., Moran N., Chaumont F. 2004. Dynamic changes in the osmotic water permeability of protoplast plasma membrane. Plant Physiol. 135:2301–2317
Nadler S.P. 1990. Effects of hypertonicity on ADH-stimulated water permeability in rat inner medullary collecting duct. Am. J. Physiol. 258:F266–F272
Nielsen S., Frokiaer J., Marples D., Kwon T.H., Agre P., Knepper M.A. 2002. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 28:205–244
Ozu M., Toriano R., Capurro C., Parisi M. 2005. Electrical parameters and water permeability properties of monolayers formed by T84 cells cultured on permeable supports. Braz J. Med. Biol. Res. 38:133-140
Parisi M., Bourguet J., Ripoche P., Chevalier J. 1979. Simultaneous minute by minute determination of unidirectional and net water fluxes in frog urinary bladder. A reexamination of the two barriers in series hypothesis, Biochim. Biophys. Acta, 556:509–523
Parisi M., Ibarra C. 1996. Aquaporins and water transfer across epithelial barriers. Braz. J. Med. Res. 29:933–939
Reid J.M., O’Neil R.G. 2000. Osmomechanical regulation of membrane trafficking in polarized cells. Biochem. Biophys. Res. Commun. 271:429–434
Reif M.C., Troutman S.L., Schafer J.A. 1984. Sustained response to vasopressin in isolated rat cortical collecting tubule. Kidney Int. 26:725–732
Ripoche P., Bourguet J., Parisi M. 1973. The effect of hyper- tonic media on water permeability of frog urinary bladder. J. Gen. Physiol. 61:110–124
Schafer J.A., Patlak C.S., Andreoly I.E. 1974. Osmosis in Cortical Collecting Tubules. A theoretical and experimental analysis of the osmotic transient phenomenon. J. Gen. Physiol. 64:201–227
Schafer J.A., Troutman S.L., Andreoli T.E. 1974. Osmosis in cortical collecting tubules. ADH-independent osmotic flow rectification. J. Gen. Physiol. 64:228–240
Strange K., Spring K.R. 1987. Cell membrane water permeability of rabbit cortical collecting duct. J. Membrane Biol. 96:27–43
Terashima M., Fujita Y., Sugano K., Asano M., Kagiwada N., Sheng Y., Nakamura S., Hasegawa A., Kakuta T., Saito A. 2001. Evaluation of water and electrolyte transport of tubular epithelial cells under osmotic and hydraulic pressure for development of bioartificial tubules. Artif. Organs 25:209–212
Toriano R., Ford P., Rivarola V., Tamarappoo B.K., Verkman A.S., Parisi M. 1998. Reconstitution of a regulated transepithelial water pathway in cells transfected with AQP2 and an AQP1/AQP2 hybrid containing the AQP2-C terminus. J. Membrane Biol. 61:141–149
Urakabe S., Handler J.S., Orloff J. 1970. Effect of hypertonicity on permeability properties of the toad bladder, Am. J. Physiol. 218:1179–1187
Valenti G., Frigeri A., Ronco P.M., D’Ettorre C., Svelto M. 1996. Expression and functional analysis of water channels in a stably AQP2-transfected human collecting duct cell line. J. Biol. Chem. 271:24365–24370
Acknowledgements
The authors thank M.A. Rivarola for his helpful assistance. This work was supported by grants from Fondo Nacional para la Ciencia y la Tecnología (FONCYT, Argentina), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina); Universidad de Buenos Aires (UBA, Argentina) and Fundación ANTORCHAS (Argentina).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chara, O., Ford, P., Rivarola, V. et al. Asymmetry in the Osmotic Response of a Rat Cortical Collecting Duct Cell Line: Role of Aquaporin-2. J Membrane Biol 207, 143–150 (2005). https://doi.org/10.1007/s00232-005-0809-9
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s00232-005-0809-9