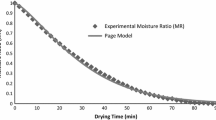
Abstract
In this study rehydration kinetics of open-sun dried okra, which dried naturally and two pre-treatment, was investigated at 25 and 50 °C. By the obtained data, parameters with respect to rehydration kinetics and diffusion mechanism were calculated. In dehydration experiments, it was determined that blanching pre-treatment has influence on the drying time and okra samples were dried at 18 h. On the contrary in rehydration experiments maximum equilibrium rehydration values were achieved with the okras dried naturally. Experimental equilibrium rehydration (R eq ), theoretical equilibrium rehydration (R max ) and diffusion coefficient (D) of okra dried naturally at 50 °C were calculated as 5.57 (g water/g dry matter), 5.96 (g water/g dry matter) and 2.17 × 10−10 (m2/s), respectively. Rehydration exponent (n) value, which is also important to identify the diffusion type of dried okra, was determined as between 0.332 and 0.383. Because of the exponent value n < 0.50, diffusion was defined as natural Fick type.







Similar content being viewed by others
Abbreviations
- M C :
-
Moisture content of dehydration (g water/g dry matter)
- m w :
-
Water content (g)
- m d :
-
Dry matter content (g)
- R C :
-
Rehydration content (g water/g dry matter)
- w t :
-
Weight of the samples at any time (g)
- w dry :
-
Dry weight (g)
- Req :
-
Experimental equilibrium rehydration (g water/g dry matter)
- EWC :
-
Equilibrium water content
- w e :
-
Rehydration values of okras at equilibrium (g water)
- dR/dt :
-
Rehydration rate
- (dR/dt)o :
-
Initial rehydration rate (g water/[g dry matter*min])
- K :
-
Rehydration rate constant (g dry matter/[g water*min])
- R max :
-
Denotes the degree of rehydration at equilibrium (g water/g dry matter)
- t :
-
Time (min)
- A :
-
The reciprocal of initial rehydration rater
- B :
-
Inverse of the degree of rehydration at equilibrium
- F :
-
Fractional uptake at time t
- k :
-
Diffusion constant
- n :
-
Diffusion exponent
- r :
-
Radius (m)
- D :
-
Diffusion coefficients (m2/s)
- R 2 :
-
Coefficient of determination
References
FAO (2013) FaoStat. Agriculture data. http://faostat3.fao.org/download/Q/QC/E. Retrieved 10 Aug 2015
Sobukola O (2009) Effect of pre-treatment on the drying characteristics and kinetics of okra (Abelmoschus esculetus (L.) Moench) slices. Int J Food Eng 5(2):9:1–9:22. doi:10.2202/1556-3758.1191
Basunia MA, Abe T (2001) Thin-layer solar drying characteristics of rough rice under natural convection. J Food Eng 47:295–301. doi:10.1016/S0260-8774(00)00133-3
Kostaropoulos AE, Saravacos GD (1995) Microwave pre-treatment for sun-dried raisins. J Food Sci 60(2):344–347. doi:10.1111/j.1365-2621.1995.tb05669.x
Saravacos GD, Marousis SN, Raouzeos GS (1988) Effect of ethyl oleate on the rate of air-drying of foods. J Food Eng 7:263–270. doi:10.1016/0260-8774(88)90008-8
Doymaz İ (2005) Sun drying of figs: an experimental study. J Food Eng 71(4):403–407. doi:10.1016/j.jfoodeng.2004.11.003
Adedeji AA, Gachovska TK, Ngadi MO, Raghavan GSV (2008) Effect of pretreatments on drying characteristics of okra. Dry Technol 26:1251–1256. doi:10.1080/07373930802307209
Doymaz İ, Kocayigit F (2011) Drying and rehydration behaviors of convection drying of green peas. Dry Technol 29:1273–1282. doi:10.1080/07373937.2011.591713
Mate JI, Zwietering M, Van’t Riet K (1999) The effect of blanching on the mechanical and rehydration properties of dried potato slides. Eur Food Res Technol 209:343–347. doi:10.1007/s002170050506
Lewicki PP (1998) Some remarks on rehydration of dried foods. J Food Eng 36:81–87. doi:10.1016/S0260-8774(98)00022-3
Sanga E, Mujumdar AS, Raghavan GSV (2000) Principles and applications of microwave drying. In: Mujumdar AS (ed) Drying technology in agriculture and food sciences. Science Publishers Inc, New Hampshire, pp 253–289
Noshad M, Mohebbi M, Shahidi F, Mortazavi SA (2011) Kinetic modeling of rehydration in air-dried quinces pretreated with osmotic dehydration and ultrasonic. J Food Process Preserv 36(5):383–392. doi:10.1111/j.1745-4549.2011.00593.x
Krokida M, Marinos-Kouris D (2003) Rehydration kinetics of dehydrated products. J Food Eng 57:1–7. doi:10.1016/S0260-8774(02)00214-5
Lee KT, Farid M, Nguang SK (2006) The mathematical modelling of the rehydration characteristics of fruits. J Food Eng 72(1):16–23. doi:10.1016/j.jfoodeng.2004.11.014
Vega-gálvez A, Notte-Cuello E, Lemus-Mondaca R, Zura L, Miranda M (2009) Mathematical modelling of mass transfer during rehydration process of Aloe vera (Aloe barbadensis Miller). Food Bioprod Process 87:254–260. doi:10.1016/j.fbp.2008.10.004
Bilbao-Sáinzet C, Andres A, Fito P (2005) Hydration kinetics of dried apple as affected by drying conditions. J Food Eng 68:369–376. doi:10.1016/j.jfoodeng.2004.06.012
Krokida MK, Philippopoulos C (2007) Rehydration of dehydrated foods. Dry Technol Int J 23(4):799–830
Krokida MK, Philippopoulos C (2005) Rehydration of dehydrated foods. Dry Technol 23(4):799–830. doi:10.1081/DRT-200054201
Maldonado S, Arnau E, Bertuzzi MA (2010) Effect of temperature and pretreatment on water diffusion during rehydration of dehydrated mangoes. J Food Eng 96:333–341. doi:10.1016/j.jfoodeng.2009.08.017
Doymaz I (2010) Effect of citric acid and blanching pre-treatments on drying and rehydration of Amasya red apples. Food Bioprod Process 88:124–132. doi:10.1016/j.fbp.2009.09.003
Markowski M, Zielinska M (2011) Kinetics of water absorption and soluble-solid loss of hot-air-dried carrots during rehydration. Int J Food Sci Technol 46:1122–1128. doi:10.1111/j.1365-2621.2011.02589.x
Ulloaa JA, Bonilla-Sanchez CR, Ortiz-Jimenez MA, Rosas-Ulloa P, Ramirez-Ramirezd JC, Ulloa-Rangel BE (2013) Rehydration properties of precooked whole beans (Phaseolus vulgaris) dehydrated at room temperature. CyTA J Food 11(1):94–99. doi:10.1080/19476337.2012.699104
Ansari S, Maftoon-Azad N, Hosseini E, Farahnaky A, Asadi Gh (2015) Modeling rehydration behavior of dried figs. J Agric Sci Technol 17:133–144
AOAC (2002) Official methods of analysis of AOAC International, 17th edn, Revision I, Gaithersburg MD, USA
Dolbow J, Eliot F, Ji H (2004) Chemically induced swelling of hydrogels. J Mech Phys Solids 52:51–84. doi:10.1016/S0022-5096(03)00091-7
Marek M, Magdalena Z (2011) Kinetics of water absorption and soluble-solid loss of hot-air-dried carrots during rehydration. Int J Food Sci Technol 46:1122–1128. doi:10.1111/j.1365-2621.2011.02589.x
Schott HJ (1992) Swelling kinetics of polymers. Macromol Sci Phys B31:1–9. doi:10.1080/00222349208215453
Wu C, Yan CY (1994) Studies of the swelling and drying kinetics of thin gelatin gel films by in situ interferometry. Macromol 27:4516–4520. doi:10.1021/ma00094a013
Doymaz I, Ismail O (2012) Modeling of rehydration kinetics of green bell peppers. J Food Process Preserv 37:907–913. doi:10.1111/j.1745-4549.2012.00724.x
Ganji F, Vasheghani-Farahani S, Vasheghani-Farahani E (2010) Theoretical description of hydrogel swelling: a review. Iran Polym J 19:375–398. http://journal.ippi.ac.ir
Ende MT, Peppas NA (1997) Transport of ionizable drugs and proteins in crosslinked poly (acrylic acid) and poly (acrylic acid-co-2-hydroxyethyl methacrylate) hydrogels II. Diffusion and release studies. J Control Release 48:47–56. doi:10.1016/S0168-3659(97)00032-1
Doymaz I (2011) Drying of green bean and okra under solar energy. Chem Ind Chem Eng Q 17(2):199–205. doi:10.2298/CICEQ101217004D
Wankhade PK, Sapkal RS, Sapkal VS (2012) Drying characteristics of okra slices using different drying methods by comparative evaluation. In: Proceedings of the world congress on engineering and computer science, vol II WCECS 2012, Oct 24–26, 2012, San Francisco, USA
Ansari S, Maftoon-Azad N, Hosseini E, Farahnaky A, Asadi GH (2015) Kinetic of color and texture changes in rehydrated figs. Tarım Bilimleri Dergisi J Agric Sci 21:108–122. doi:10.15832/tbd.47774
Turhan M, Sayar S, Gunasekaran S (2002) Application of Peleg model to study water absorption in chickpea during soaking. J Food Eng 53:153–159. doi:10.1016/S0260-8774(01)00152-2
Demirbüker D, Arcan I, Tokatli F, Yemenicioglu A (2005) Effects of hot rehydration in the presence of hydrogen peroxide on microbial quality, texture, color and antioxidant activity of cold stored intermediate moisture sun-dried figs. Food Sci 70:153–159. doi:10.1111/j.1365-2621.2005.tb07143.x
Lee SJ, Kim SS, Lee YM (2000) Interpenetrating polymer network hydrogels based on poly (ethylene glycol) macromer and chitosan. Carbohydr Polym 41:197–205. doi:10.1016/S0144-8617(99)00088-0
Apar DK, Demirhan E, Ozbek B, Dadali G (2009) Rehydration kinetics of microwave-dried okras as affected by drying conditions. J Food Process Preserv 33(5):618–634. doi:10.1111/j.1745-4549.2008.00277.x
Ritger PL, Peppas NA (1987) Transport of penetrants in the macromolecular structure of coals. 4. Models for analysis of dynamic penetrant transport. Fuel 66:815–826. doi:10.1016/0016-2361(87)90130-X
Peppas NA, Brannon-Peppas L (1994) Water diffusion and sorption in amorphous macromolecular systems and foods. J Food Eng 22:189–210. doi:10.1016/0260-8774(94)90030-2
Bello M, Tolaba MP, Suarez C (2004) Factors affecting water uptake of rice grain during soaking. LWT Food Sci Technol 37(8):811–816. doi:10.1016/j.lwt.2004.02.014
Calzetta Resio AN, Aguerre RJ, Suarez C (2003) Study of some factors affecting water absorption by amaranth grain during soaking. J Food Eng 60(4):391–396. doi:10.1016/S0260-8774(03)00062-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gökçe Kocabay, Ö., İsmail, O. Investigation of rehydration kinetics of open-sun dried okra samples. Heat Mass Transfer 53, 2155–2163 (2017). https://doi.org/10.1007/s00231-017-1972-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-017-1972-0