Abstract
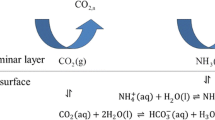
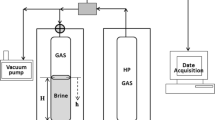
Interfacial mass transfer of \(\hbox {NH}_3\) and \(\hbox {CO}_2\) are important in processes as diverse as \(\hbox {NH}_3\) emission from animal manure and gas scrubbing for removal of carbon dioxide. Predicting transfer rates is complicated by bidirectional interactions between solution pH and emission rates, which may be affected by physical, chemical, and biological processes. We studied the effects of \(\hbox {CO}_2\) hydration kinetics and evaporative convection on the development of pH profiles in solutions undergoing simultaneous emission of \(\hbox {NH}_3\) and \(\hbox {CO}_2\). Profiles of pH were measured at a 0.1 mm resolution over 15 h, and interpreted using a reaction-transport model. Under high humidity, surface pH increased quickly (>0.2 units in 8 min) and an increase gradually extended to deeper depths. An increase in \(\hbox {CO}_2\) hydration and carbonic acid dehydration rates by addition of carbonic anhydrase increased the elevation of surface pH and the depth to which an increase extended, due to an increase in \(\hbox {CO}_2\) emission. Results show that unless carbonic anhydrase is present, the equilibrium approach typically used for modeling interfacial transport of \(\hbox {CO}_2\) and \(\hbox {NH}_3\) will be inaccurate. Evaporation and resulting convection greatly increased mass transfer rates below an apparent surface film about 1 mm thick. Emission or absorption of \(\hbox {CO}_2\) can produce steep gradients in pH over small distances (<0.5 to >20 mm) in systems with and without convective mixing, and the resulting surface pH, in turn, strongly affects \(\hbox {NH}_3\) transfer. Both convection and the rate of hydration/dehydration reactions are likely to affect pH profile development and rates of \(\hbox {NH}_3\) and \(\hbox {CO}_2\) transfer in many systems. Accurately predicting mass transfer rates for these systems will require an understanding of these processes in the systems.




Similar content being viewed by others
Abbreviations
- D :
-
Lumped diffusion coefficient of all aqueous species in water (\(\hbox {m}^2\hbox { s}^{-1}\))
- \(h_m\) :
-
Surface convection coefficient for mass transfer of \(\hbox {NH}_3\) (m s\(^{-1}\))
- c :
-
Constant by which rates of \(\hbox {CO}_2\) hydration and carbonic acid dehydration are multiplied to simulate the effect of carbonic anhydrase (dimensionless, \({\ge }1\))
- \(\delta\) :
-
Thickness of a diffusion-dominated boundary layer within the liquid phase (mm)
- k :
-
Lumped convection/dispersion coefficient of all aqueous species in water below \(\delta\) (\(\hbox {m}^2\hbox { s}^{-1}\))
- RH:
-
Relative humidity (% of saturation concentration)
- CA:
-
Carbonic anhydrase
References
Denmead O, Freney J (1992) Transfer coefficients for water-air exchange of ammonia, carbon dioxide and methane. Ecol Bull 42:31–41
Kirk G, Rachhpal-Singh (1992) Simultaneous transfer across an air–water interface of gases that interact through acid–base reactions. Atmos Environ A Gen Top 26(9):1651. doi:10.1016/0960-1686(92)90064-R
Sommer S (2013) Ammonia volatilisation from livestock slurries and mineral fertilisers. University Press of Southern Denmark, Odense
Campos JC, Moura D, Costa AP, Yokoyama L, da Fonseca Araujo FV, Cammarota MC, Cardillo L (2013) Evaluation of pH, alkalinity and temperature during air stripping process for ammonia removal from landfill leachate. J Environ Sci Health A Toxic Hazard Substan Environ Eng 48(9):1105. doi:10.1080/10934529.2013.774658
Zhuang Q, Pomalis R, Zheng L, Clements B (2011) Ammonia-based carbon dioxide capture technology: issues and solutions. Energy Procedia 4:1459. doi:10.1016/j.egypro.2011.02.012
Hafner SD, Montes F, Alan Rotz C (2013) The role of carbon dioxide in emission of ammonia from manure. Atmos Environ 66:63. doi:10.1016/j.atmosenv.2012.01.026
Petersen V, Markfoged R, Hafner SD, Sommer SG (2014) A new slurry pH model accounting for effects of ammonia and carbon dioxide volatilization on solution speciation. Nutr Cycl Agroecosyst. doi:10.1007/s10705-014-9637-6
Kern DM (1960) The hydration of carbon dioxide. J Chem Educ 37:14
Kumar RSS, Ferry JG (2014) Prokaryotic carbonic anhydrases of Earth’s environment. Sub-Cell Biochem 75:77
Moroney JV, Bartlett SG, Samuelsson G (2001) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant Cell Environ 24(2):141. doi:10.1111/j.1365-3040.2001.00669.x
Henry RP, Swenson ER (2000) The distribution and physiological significance of carbonic anhydrase in vertebrate gas exchange organs. Respir Physiol 121(1):1. doi:10.1016/S0034-5687(00)00110-9
Volino RJ, Smith GB (1999) Use of simultaneous IR temperature measurements and DPIV to investigate thermal plumes in a thick layer cooled from above. Exp Fluids 27(1):70. doi:10.1007/s003480050330
Elzhov TV, Mullen KM, Spiess AN, Bolker B (2013) minpack.lm: R interface to the Levenberg–Marquardt nonlinear least-squares algorithm found in MINPACK, plus support for bounds. http://CRAN.R-project.org/package=minpack.lm. R package version 1.1-8
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Liss P, Slater PG (1974) Flux of gases across air–sea interface. Nature 247(5438):181
Willmott C (1982) Some comments on the evaluation of model performance. Bull Am Meteorol Soc 63(11):1309
Katsaros KB, Liu WT, Businger JA, Tillman JE (1977) Heat thermal structure in the interfacial boundary layer measured in an open tank of water in turbulent free convection. J Fluid Mech 83(02):311. doi:10.1017/S0022112077001219
Bennett J, Rathbun R (1972) Reaeration in open-channel flow. Geological Survey Professional Paper 737, United States Government Printing Office, Washington, DC
Bukhari SJK, Siddiqui MHK (2007) Characteristics of air and water velocity fields during natural convection. Heat Mass Transf 43(5):415. doi:10.1007/s00231-005-0072-8
Fan LS (1989) Gas–liquid–solid fluidization engineering. Butterworth-Heinemann, Boston
Williams GR (1983) The rate of hydration of carbon dioxide in natural waters. Ecol Bull 35:281–289
Goldman JC, Dennett MR (1983) Carbon dioxide exchange between air and seawater: no evidence for rate catalysis. Science 220(4593):199. doi:10.1126/science.220.4593.199
Matthews BJH (1999) The rate of air–sea CO\(_2\) exchange: chemical enhancement and catalysis by marine microalgae. Ph.D. thesis, University of East Anglia, Norwich
Madigan MT, Martinko JM, Stahl D, Clark DP (2010) Brock Biol Microorg, 13th edn. Benjamin Cummings, San Francisco
Acknowledgments
Funding was provided by Grønt udviklings- og demonstration program (Gylle-IT), Ministeriet for Fødevarer, Landbrug og Fiskeri—NaturErhvervstyrelsen. We thank Lars B. Pedersen, Preben Sørensen, and Niels Peter Revsbech for constructing the pH and temperature electrodes. Lastly, we thank Steen Bennike Mortensen (NovoZymes) for providing us with the carbonic anhydrase.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hafner, S.D., Sommer, S.G., Petersen, V. et al. Effects of carbon dioxide hydration kinetics and evaporative convection on pH profile development during interfacial mass transfer of ammonia and carbon dioxide. Heat Mass Transfer 53, 1335–1342 (2017). https://doi.org/10.1007/s00231-016-1910-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-016-1910-6