Abstract
Nanofluids have recently attracted researches’ attention as a new generation of heat-transferring fluids used in heat exchangers and for energy storage. Also aluminium nitride is commonly known for its considerable heat conductivity, as high as 320 W/(m K). Because of that, the compound might be a preferable dispersed phase of heat-transferring fluids. This presented studies are focused on nano-AlN–poly(propylene glycol) dispersions which can be applied as potential cooling fluids. The rheological response of the suspensions on shearing and their thermal conductivity in the function of solids concentration and temperature were measured and discussed. The most desired result of the studies is to produce dispersions with Newtonian-like flow at increased temperature and at higher shear rate. All the aforementioned parameters conjugated with significant thermal conductivity of such nanofluids could predispose them to be used as effective cooling media.
Similar content being viewed by others
1 Introduction
Nanofluids are produced when spherical nanoparticles, nanorods, nanotubes or nanofibres are dispersed in various base fluids [1, 2]. These particular media are usually obtained using one- or two-step synthesis method [1, 3]. Nanofluids have an particular applicable potential due to their highly enhanced thermal properties [2, 4–8]. Additionally, nanofluids usually represent an improved time–stability compared with some conventional heat-transferring fluids with submicrometric or micrometric particles [2, 9, 10]. These properties result from the size effect of nanoparticles and their Brownian motion in liquid media. Nanofluids can also flow smoothly through microchannels without any clogging effect, so the size of heat transfer systems can be reduced [1, 6, 10].
Because of these properties the usage of nanofluids in an industrial process may create energy savings, reduce process time, raise thermal rating and lengthen the working life of equipment [11]. On the other hand the health risks and handling difficulty of nanofluids are not yet completely understood. Integrating the nanofluids into existing forced-convection liquid cooling systems might create concerns of piping erosion, component damage, and nanoparticle settling or agglomeration [12]. Application of nanofluids as efficient coolants in advanced industry depends not only on their high thermal conductivity, but also critically depends on their rheological properties like viscosity, viscoelasticity, etc. [6, 8, 9, 13, 14]. For certain applications, like heat-microexchangers, high thermal conductivity is required with a minimal nanofluid viscosity. However, until now, much less attention has been paid to both theoretical and experimental investigations on their rheological properties than on their thermal conductivity studies.
Nanofluids industry uses varied metallic and ceramic powders which are capable to heat transport in flow conditions [2, 6, 9, 15, 16]. One of the powders is aluminium nitride (AlN), which has recently paid much attention [4, 7, 8, 17]. It has been applied as an important ceramic substrate material due to its high thermal conductivity, low thermal expansion coefficient, low dielectric constant, good mechanical strength, thermal stability, non-toxicity, stable crystal structure and relatively low cost. These unique properties have made AlN an attractive material for high-tech industrial applications such as electrical packaging and heat sinks. Aluminium nitride is an attractive material for semiconductor devices because of its high thermal conductivity, low dielectric constant, thermal expansion coefficient similar to silicon, and excellent mechanical strength. Nevertheless, these AlN properties are very sensitive to impurities, which may be harmful in the sintering process [7, 17].
Moreover, it is difficult to obtain fully dense AlN sintered pieces due to its low diffusion coefficient and its high sintering temperature (2173 K). The sintering temperature of AlN can be decreased by making the particles smaller, leading to an increase in their surface area. Therefore, the current scientific challenge is to synthesize nano-sized AlN powder of high purity. In general, AlN could be fabricated in a variety of methods, such as carbothermal nitridation, direct nitridation of aluminium powders, chemical vapour deposition and plasma processes, among which carbothermal nitridation and direct nitridation of aluminium powders have been the most widely used in the industrial production [18].
Commercially available poly(propylene glycol) 2000 (abbrev. PPG 2000) from Acros Organics was used as the dispersive phase of the studied nanofluids. This colour- and odourless viscous liquid is commonly used as an ingredient in many household products and cosmetics. PPG is also widely applied in power generation industry, chemical production, air-conditioning, transportation and microelectronics [19]. The researchers community more often takes into account ethylene or poly(ethylene glycol) as a dispersive phase in nanofluids instead of propylene or poly(propylene glycol) [20–22]. Ethylene glycol does not transport as much of heat as propylene glycol. Because of that more fluid must be circulated to transfer the same amount of energy and therefore pumps volume must be increased.
The first purpose of the research was to study some rheological properties of nano-AlN–PPG systems. In order to demonstrate the behavior of the dispersions at different conditions, solid phase loading, shear rate and temperature were the variables. Subsequently, the thermal response of suspensions containing various solids loading was measured at different temperatures and discussed. Since the systems consist of suitable ratio of solid/liquid phase and they have some advantageous rheological properties and thermal response, they could have high potential to be applied in cooling industry.
2 Materials and methods
2.1 Materials
Nanometric aluminium nitride was purchased from Sigma-Aldrich (Aluminium nitride nanopowder, <100 nm particle size) [23].
The AlN powder was used in as-received state (no thermal or chemical treatment had been applied). Figure 1 shows TEM micrograph of the powder; according to its manufacturer; AlN grains are well-dispersed and their average particle size is well below 100 nm.
TEM micrograph of nano-AlN given by Sigma Aldrich [23]
Non-aqueous dispersive phase preserves AlN particles from the reaction with water [7, 17]. Therefore, poly(propylene glycol) with an average molecular weight of ca. 2000 (Acros Organics) was used as the dispersive phase for nano-AlN. The liquid is commonly designated as PPG 2000. The recent studies have confirmed usefulness of that particular solvent to obtain AlN suspensions of some advantageous features [7, 17]. PPG 2000 liquid is thermally resistant and highly stable, its vapour pressure at 20 °C is <0.01 mmHg. Due to its hygroscopic properties, PPG 2000 should be kept sealed to avoid steam imbibition. Density of PPG 2000 at 25 °C is 1.005 g/cm3; its kinematic viscosity is 3 × 10−4 m2/s at 25 °C.
2.2 Aluminum nitride characteristics
SEM observations of nano-AlN combined with X-ray microanalysis were carried out by using ultra high-resolution Nova Nano SEM 200 Scanning Electron Microscope (FEI EUROPE COMPANY) attached with EDS analyzer by EDAX. TEM analysis of nano-AlN powder was conducted by using of FEI Tecnai TF-20 X-Twin (FEG) transmission electron microscope. Accelerating voltage during the analysis was 200 kV. Isopropanol dispersion of nano-AlN had been ultrasonificated for 30 min and then placed on copper mesh with carbon surface film. X-ray diffraction (Cu Kα radiation) was conducted on X’Pert Pro, Panalytical equipment. Bulk density of nano-AlN powder was measured on helium pycnometer AccuPyc 1330 from Micromeritics. Specific surface area SSABET was determined on the basis of adsorption/desorption isotherm of nitrogen molecules at the temperature of liquid nitrogen. The equipment used for the study was ASAP 2010 device from Micromeritics. Particle size distribution analysis was carried out by using of Metasizer 2000 device (Malvern Instruments). PSD measurements of nano-AlN were made in isopropanol. Concentration of the powder was 0.0088 vol%. The size range of the PSD measurement was 0.020–2000.000 μm. The average value of seven measurements was taken into account.
2.3 Viscosity measurements of nano-AlN_PPG 2000 systems
Rheological measurements of nano-AlN_PPG 2000 systems were conducted by using of Physica MCR-301 (Anton Paar) rotational rheometer attached with two parallel plates (Θ = 50 mm, measuring gap = 0.500 mm). Increasing and decreasing values of shear rate (0.1–1000/s) was applied in order to determine viscous behavior of the suspensions. The measurements were taken at the temperature range of 20–50 °C with the temperature step 10 °C (±0.2 °C).
2.4 Preparation of nano-AlN_PPG 2000 dispersions
AlN–PPG dispersions were prepared via two-step synthesis. TD 100 laboratory dissolver (Pendraulik-Teja) was equipped with a mechanically modulated dispersive disk. The powder was incrementally added to PPG continuous phase, and then the suspension was homogenized. Subsequently, the dispersions were stirred for 40 min at 3000 RPM. It had been assumed that such an intensive stirring broke some AlN agglomerates. The as-obtained suspensions were actually highly flowable and definitely homogenous. Solid phase loading in nano-AlN–PPG 2000 suspensions was 0.1, 0.5, 5, 10, 15 and 20 vol%.
2.5 Thermal conductivity measurements
Laser flash analysis (abbrev. LFA) was used to measure thermal conductivity of nano-AlN–PPG 2000 dispersions. AlN dispersions were placed into platinum holder of LFA 427 apparatus (Netzsch Company). Its measuring range covers thermal conductivity from 0.1 to 2000 W/(m K). Thermal diffusivity of the samples was measured at 20, 40, 60 and 80 °C. Proteus analysis software was used to converse values of thermal diffusivity into thermal conductivity. Thermal conductivity was designated as k (W/(m K)). Thermal conductivity enhancement (TCE, %) was computed by comparing thermal conductivity of AlN–PPG dispersion to its base poly(propylene glycol) liquid is following as:
where k thermal conductivity of AlN–PPG dispersion (W/(m K)), k0 thermal conductivity of PPG (W/(m K)).
The maximum tolerance of thermal conductivity measurement in the case of PPG liquids and AlN–PPG dispersions is ca. 4 %. For statistical reasons three laser shots were measured for each temperature and average of this three was calculated.
3 Results and discussion
3.1 Materials characteristics
Morphological features of nano-AlN powder are depicted in Figs. 2, 3, 4, 5 and 6. SEM micrograph reveals loosely agglomerated grains with the wide range of the primary particle size: from dozens of nanometers to several micrometers. The particles are strongly irregular, the finest grains are mostly spherical, but the largest ones are polyhedral crystallites. SEM micrograph of nano-AlN provided by its manufacturer (Fig. 2) probably shows the finest fraction of the particles.
Four columnar graphs in Fig. 3 show an atomic % (at.%) of elements detected by X-ray microanalysis attachment (EDS attachment).
Four nano-AlN grains had undergone EDS analysis and the studies detected elemental aluminium, nitrogen and oxygen. The quantities of the elements presented in Fig. 3 are as follows: 63, 51–80, 34. at.% of Al, 14, 1–27.01 at.% of N, 4, 38–9, 48 at.% of O. Oxygen contaminant probably originated from hydrolysed surface of nano-AlN. The powder surface is highly developed and readily hydrolyses to Al(OH)3·nH2O. Although the manufacturer of the powder does not inform about it, oxygen can be the residue from AlN synthesis route. Nevertheless, oxygen contaminant remains low and constant in the studied grains.
TEM micrograph of nano-AlN powder is depicted in Fig. 4. The picture strongly varies from the manufacturer’s TEM micrograph (see Fig. 1). As contrasted with AlN in Fig. 1, the powder in Fig. 4 has a wide particle size distribution; nevertheless, the careful analysis of the TEM micrograph reveals that some of the largest entities are in deed aggregates, not single crystallites. Assessing the Fig. 4, it can be clearly stated that the powder particles are not well below 100 nm as it has been presented by its manufacturer.
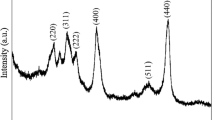
The results of X-ray analysis of nano-AlN powder are depicted in Fig. 5. The signals are actually characteristic for aluminium nitride; only some negligible and barely visible traces of other phases can be spotted. Nevertheless; their origin and nature is practically impossible to determine. The XRD pattern shows practically no sign of nanosized entities in the powder. The peaks are narrow and well-shaped; none of them shows a characteristic broadening of the signal of the finest particles.
Pycnometric density of AlN powder was 3.24 g/cm3, which is close to theoretical density of aluminium nitride i.e. 3.26 g/cm3.
Specific surface area of the powders calculated on the basis of BET isotherm was 1.99 m2/g. The value is rather low for a ceramic powder; an average particle size computed from SSABET and density (3.24 g/cm3) was 0.93 µm. The value of dBET strongly differs from an average particle size claimed by the manufacturer of the powder. This evident discrepancy can be due to agglomeration of AlN nanopowder. Nitrogen molecules adsorb on agglomerates and consequently they are interpreted as the primary particles of the powder.
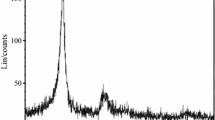
Diluted AlN–isopropanol suspension was prepared to determine particle size distribution of as-received AlN powder; the graph in Fig. 6 illustrates the issue.
It can be read out that d(0.1) is ca. 2.7 μm, d(0.5) = 6.0 μm and d(0.9) = 20.1 μm. Once again, the analysis shows a non-nanometric feature of the “nano-”AlN. Most probably, its particles strongly agglomerate in isopropanol, in spite of strong dilution of the suspension. Nevertheless, this unimodal particle size distribution was considered as sufficient for the investigation. Minor peak 0.3 vol% in 200 μm could be caused by technological process of the powder production or by hydrolysis in moist air during storage. Very high surface energy of the nanopowder can lead to agglomeration of the particles which is very difficult to reverse by sonication.
3.2 Solid phase loading and temperature impact on AlN–PPG 2000 suspensions
Before viscosity measurements of AlN suspensions had been taken, viscous behaviour of their dispersive phase was studied. The results of the studies have confirmed that PPG 2000 liquid is a Newtonian fluid. The viscosity of the fluid decreases with an increasing temperature; at 20 °C it is ca. 100 mPa s whereas at 60 °C–20 mPa s [7, 17]. The powder concentration influence on AlN–PPG 2000 viscosity at 20 °C is presented in Fig. 7. The viscosity of the system increased with the increasing solid phase loading at the whole shear range, which is similar to literature [9, 24].
All dispersions present very slight shear thinning behaviour at the lowest shear rates. When shear rate exceeds ca. 200/s, the shear thinning behaviour decays and transforms into Newtonian-like flow and an “equilibrium viscosity” can be read out. The viscosity of 20 vol% AlN–PPG 2000 system at 1000/s is ca. three times higher than in the case of 0.5 vol% AlN–PPG 2000 at 1000/s (1.32 vs. 0.47 Pa s).
The increase of suspension viscosity with an increasing solid phase loading is an obvious phenomenon in such systems. Nevertheless, such measurements are necessary to optimize solids concentration in as-received dispersions.
The viscosity of the same nano-AlN–PPG 2000 systems were also measured at 50 °C (see Fig. 8). Their Newtonian-like flow is preceded by shear thinning behaviour as it has been noticed at 20 °C. The viscosity of AlN–PPG 2000 systems is strongly temperature-dependent; it decreases form ca. 1.32 Pa s (20 vol%, 20 °C, 1000/s) to 0.26 Pa s (20 vol%, 50 °C, 1000/s). That strong temperature-dependency is definitely advantageous for these potentially cooling suspensions. When affected by any transported heat, the suspensions flow freely cause less resistance. Moreover, it is also worth to emphasize that the AlN–PPG 2000 dispersions are not shear thickened. The feature rules out such a dispersion from any flow-associated applications.
3.3 Thermal conductivity of AlN–PPG liquids
It has been previously studied and discussed that thermal conductivity of PPG 2000 liquid is moderate and slightly increases with temperature [8, 14]. The values of PPG 2000 thermal conductivity range from ca. 0.18 to 0.19 W/(m K) (at the temperature range from 20 up to 80 °C).
Generally, thermal conductivity of nano-AlN suspensions (0.1–20 vol% of ceramic powder, temperature range 20–80 °C) do not exceed 0.40 W/(m K) (Fig. 9). Generally, the addition of the AlN powder to PPG 2000 increases suspensions conductivity—when the powder is added, thermal conductivity of the suspensions increases up to 106 % of pure PPG 2000 (Fig. 10). What is surprising, is that for the 0.1 vol% of AlN the decrease of thermal conductivity of suspension was noticed. It might be caused by the temperature impact on AlN suspensions with a constant solids loading is difficult to specify; thermal conductivity seems not to be temperature-dependant (Fig. 9).
Figure 10 shows that thermal conductivity enhancement strongly increases with increasing solid phase concentration ranging from ca. 18 to nearly 106 %. The highest values of thermal conductivity were measured for 20 vol% AlN–PPG 2000 fluid; TCE parameter in this case nearly exceeded 100 % in all temperature ranges. More specific trend is ready to observe when solids loading increases at constant temperature—see Fig. 11. Thermal conductivity of AlN suspensions increases almost linearly at 40 and 80 °C as solids loading increases.
The phenomenon of the increasing thermal conductivity with the increasing solids loading points on the crucial role of AlN concentration in heat transfer in the suspensions bulk. The AlN loading changes thermal conductivity more evidently than any temperature ramp—compare Figs. 9 and 11—it can be assumed that in the temperature range between 20 and 80 °C, temperature itself does not modify the way of heat transfer. Most probably, the heat-transferring effect of AlN structures is not temperature—but solids loading—dependant; such a phenomenon has been previously noticed and discussed [7, 17].
3.4 Comparison of measured thermal conductivity of nanoparticle: fluid mixtures with theoretical results
Obtained thermal conductivity data for four different solid loadings (5, 10, 15 and 20 vol%) were compared with theoretical calculations. Four models were considered as follow.
Maxwell model can be applied to statistically homogeneous and low-volume fraction liquid–solid suspensions with randomly dispersed, uniformly sized and non-interacting spherical particles [25]:
where k p and k f is particle’s and fluid thermal conductivity, k is nanofluid conductivity and ϕ is volume fraction of nanoparticles.
Chen’s [26] modified Hamilton-Crosser model for aggregates of nanoparticles:
where k p were replaced by k a —aggregates thermal conductivity and \(\phi_{a} = \phi (r_{a} /r)^{3 - D}\) is effective volume of aggregates with r a and r being the aggregates and particles radii, D is fractal index of aggregates and has average of 1.8. Aggregates thermal conductivity can be determined from Bruggeman’s model:
where \(\phi_{i} = (r_{a} /r)^{D - 3}\) is solid volume concentration of agglomerates. Hashin–Shtrikman model [27] is widely used to estimate upper and lower bounds of effectivethermal conductivity using formula:
where lower bound is identical to Maxwell model and upper bound corresponds to nanocluster matrix with spherical inclusions of fluid regions. A second order formulation extended from Maxwell’s model developed by Jeffry is as follows [28]:
where \(\alpha = k_{p} /k_{f}\) is particle to fuid thermal conductivity ratio and \(\beta = (\alpha - 1)/(\alpha + 2)\).
In the calculations, the thermal conductivity of AlN nanoparticles is taken as 280 W/m K and thermal conductivity of PPG 2000 according as temperature is in the range of 0,183 and 0,189 W/m K. The particles radii is 1 µm and aggregates 5 µm.
From Fig. 12, it can be clearly seen that the measured thermal conductivity is in most cases greater than the value calculated using the thermal conductivity theories. The mechanism of the great instability of thermal conductivity of 15 vol% AlN–PPG 2000 versus temperature is still unknown. The difference between obtained data and theoretical calculations is caused probably by the fact, that the theoretical models are focused mainly on statistically homogeneous, isotropic composite materials with randomly dispersed spherical particles having uniform particle size. This suggests that all of the current models, which only account for the differences between thermal conductivity of particles and fluids, are not sufficient to explain the energy transfer processes in nanoparticle–fluid mixtures [29].
4 Conclusions
The paper presents the studies on concentrated nano-AlN–PPG 2000 dispersions as potentially applicable coolants. Nevertheless, the used powder is not strictly “nano” just like its manufacturer ensures. Additionally, the studies clearly indicates that as-used AlN powder is strongly agglomerated. All measured dispersions have Newtonian-like flow, preceded by minor shear thinning behaviour. No dilatancy effect was observed, what is considered to be an advantageous feature of the AlN suspensions. It has been demonstrated that the viscosity of the nanofluids definitely increases with an increase of solids concentration. Moreover, the viscosity of the dispersions decreases ca. five times with an increase of temperature at the range of 20–50 °C.
The studies on thermal conductivity indicate that the parameter increases almost linearly up with an increase of solids concentration in its base fluid. The values of thermal conductivity of “nano-”AlN–PPG 2000 suspensions are mostly lower than thermal conductivities of micro-AlN–PPG 425/2000 dispersions [7, 17]. The comparison of theoretical calculation with measured data showed a big difference in thermal conductivity. Theoretical calculations are focused mainly on statistically homogeneous, isotropic composite materials with randomly dispersed spherical particles having uniform particle size which is significantly simplified. Because of early stage of laboratory trials and steady conditions of thermal conductivity measurements there is no clear answer of advantages that AlN–PPG 2000 suspensions have over other coolants. Such system might behave differently on a macro scale and under flow condition.
References
Keblinski P, Eastman JA, Cahill DG (2005) Nanofluids for thermal transport. Mater Today 8:36–44
Philip J, Shima PD (2012) Thermal properties of nanofluids. Adv Colloid Interface Sci 183–184:30–45
Wen DS, Lin GP, Vafaei S, Zhang K (2009) Review of nanofluids for heat transfer applications. Particuology 7:141–150
Choi C, Yoo HS, Oh JM (2008) Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr Appl Phys 8:710–712
Hu P, Shan WL, Yu F, Chen ZS (2008) Thermal conductivity of AlN–ethanol nanofluids. Int J Thermophys 29:1968–1973
Saidur R, Leong KY, Mohammad HA (2011) A review on applications and challenges of nanofluids. Renew Sustain Energy Rev 15:1646–1668
Wozniak M, Danelska A, Rutkowski P, Kata D (2013) Thermal conductivity of highly loaded aluminium nitride–poly(propylene glycol) dispersions. Int J Heat Mass Transf 65:592–598
Yu W, Xie H, Li Y, Chen L (2011) Experimental investigation on thermal conductivity and viscosity of aluminium nitride nanofluids. Particuology 9:187–191
Mahbubul IM, Saidur R, Amalina MA (2012) Latest developments on the viscosity of nanofluids. Int J Heat Mass Transf 55:874–885
Das SK, Choi SUS, Patel HE (2006) Heat transfer in nanofluids—a review. Heat Transfer Eng 27:3–19
Kazi SN (2012) An overview of heat transfer phenomena. InTech, Sivashanmugam P (ed) Chapter 14 Application of nanofluids in heat transfer
Schroeder SP (2010) Nanofluids in a forced-convection liquid cooling system—benefits and design challenges. In: 12th IEEE intersociety conference on thermal and thermomechanical phenomena in electronic systems (ITherm)
Yang JC, Li FC, Zhou WW, He YR, Jiang BC (2012) Experimental investigation on the thermal conductivity and shear viscosity of viscoelastic-fluid-based nanofluids. Int J Heat Mass Transf 55:3160–3166
Mondragon R, Julia JE, Barba A, Jarque JC (2012) Determination of the packing fraction of silica nanoparticles from the rheological and viscoelastic measurements of nanofluids. Chem Eng Sci 80:119–127
Teng TP, Hung YH, Teng TC, Mo HE, Hsu HG (2010) The effect of alumina/water nanofluid particle size on thermal conductivity. Appl Therm Eng 30(14–15):2213–2218
Sharma P, Baek IH, Cho T, Park S, Lee KB (2011) Enhancement of thermal conductivity of ethylene glycol based silver nanofluids. Powder Technol 208:7–19
Wozniak M, Danelska A, Kata D, Szafran M (2012) New anhydrous aluminium nitride dispersions as potential heat-transferring media. Powder Technol 235:717–722
Kim K (2005) Plasma synthesis and characterization of nanocrystalline aluminium nitride particles by aluminium plasma jet discharge. J Cryst Growth 283:540–546
Propylene Oxide/Propylene Glycol sector group Cefic. http://www.propylene-glycol.com/uploads/PropyleneGlycolAdvocacybrochure.pdf. Accessed 20 July 2014
Bley H, Fussnegger B, Bodmeier R (2010) Characterization and stability of solid dispersions based on PEG/polymer blends. Int J Pharm 2:165–173
Biswal S, Sahoo J, Murthy PN, Giradkar RP, Avari JG (2008) Enhancement of dissolution rate of gliclazide using solid dispersions with polyethylene glycol 6000. AAPS PharmSciTech 9(2):563–570
Sahu RK, Somashekhar SH, Manivannan PV (2013) Investigation on copper nanofluid obtained through micro electrical discharge machining for dispersion stability and thermal conductivity. Procedia Eng 64:946–955
Sigma Aldrich Company, Specification of Aluminum nitride nanopowder, <100 nm particle size. http://www.sigmaaldrich.com/catalog/product/aldrich/593044?lang=en®ion=BE, http://www.sigmaaldrich.com/Graphics/COfAInfo/SigmaSAPQM/SPEC/59/593044/593044-BULK_______ALDRICH__.pdf. Accessed 20 July 2014
Chen H, Ding Y, Tan C (2007) Rheological behaviour of nanofluids. New J Phys 9:367
Maxwell JC (1881) A treatise on electricity and magnetism, vol 1, 2nd edn. Clarendon Press, Oxford
Chen H, Witharana S, Jin Y, Kim C, Ding Y (2009) Predicting thermal conductivity of liquid suspensions of nanoparticles (nanofluids) based on rheology. Particuology 7:151–157
Hashin Z, Shtrikman S (1962) A variational approach to the theory of the effective magnetic permeability of multiphase materials. J Appl Phys 33:3125
Jeffrey DJ (1973) Conduction through a random suspension of spheres. Proc R Soc Lond Ser A 335(1602):355–367
Wang X, Xu X (1999) Thermal conductivity of nanoparticle–fluid mixture. J Thermophys Heat Transf 13:4
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wozniak, M., Rutkowski, P. & Kata, D. Rheological properties and thermal conductivity of AlN–poly(propylene glycol) suspensions. Heat Mass Transfer 52, 103–112 (2016). https://doi.org/10.1007/s00231-015-1597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-015-1597-0