Abstract
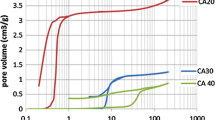
The effects on mass transference phenomena due textural changes of monolithic carbon aerogels were studied by hexane adsorption. The monolithic carbon aerogels were prepared after carbonization of the organic aerogels obtained by resorcinol–formaldehyde polymerization, using p-toluenesulfonic acid (acid-catalyst) and sodium carbonate catalysts (basic-catalyst). Internal texture was modified by CO2 activation. The characterization by gas adsorption showed that the monolithic carbon aerogels presents a bi-modal pore size distribution with presence of both microporous and mesoporous. It was shown that the activation process of monolithic carbon aerogels increases their micropore volume bigger than the other one acid-catalyst aerogel. The mesopores volume in the carbon aerogels plays an important role on mass transport mechanism. The samples with presence of significant mesopore volume present a lower height of mass transfer zone than others less mesopore volume; therefore better efficiency of adsorption in mass transfer zone in dynamic adsorption. The breakthrough curve methodology proposed in this work has allowed finding a relationship between the structural parameters and dynamic adsorption variables, which opens new approaches for measuring textural parameters of material.






Similar content being viewed by others
Abbreviations
- D :
-
Pore diameter (Nm)
- E 0 :
-
Micropore adsorption energy (J mol−1)
- H MTZ :
-
Height mass transfer zone (cm)
- P/P0 :
-
Relative pressure
- R :
-
Gas constant
- S:
-
Specific surface area (m2 g−1)
- T :
-
Temperature (K)
- T :
-
Time (min)
- V :
-
Volume (cm3)
- W :
-
Standard micropore volume (cm3 g−1)
- W 0 :
-
Total standard micropore volume (cm3 g−1)
- XH :
-
Volume adsorbed of hexane (cm3 g−1)
- β :
-
Dubinin affinity coefficient
- ϕ :
-
Fractional capacity
- BET:
-
Brunauer–Emmett–Teller
- Benz:
-
Benzene
- brk:
-
Breakthrough
- Hex:
-
Hexane
- DR:
-
Dubinin–Radushkevich
- Meso:
-
Mesopore
- Micro:
-
Micropore
- Sto:
-
Stoichiometric
References
Yi FY, Lin XD, Chen SX, Wei XQ (2009) Adsorption of VOC on modified activated carbon fiber. J Porous Mater 16(5):521–526. doi:10.1007/s10934-008-9228-5
Fairen-Jimenez D, Carrasco-Marin F, Moreno-Castilla C (2007) Adsorption of benzene, toluene, and xylenes on monolithic carbon aerogels from dry air flows. Langmuir 23(20):10095–10101. doi:10.1021/1a701458h
Díaz E, Ordóñez S, Vega A, Coca J (2005) Catalytic combustion of hexane over transition metal modified zeolites NaX and CaA. Appl Catal B 56(4):313–322
Ordóñez S, Bello L, Sastre H, Rosal R, Díez FV (2002) Kinetics of the deep oxidation of benzene, toluene, n-hexane and their binary mixtures over a platinum on [gamma]-alumina catalyst. Appl Catal B 38(2):139–149
Díaz E, Ordóñez S, Vega A, Coca J (2005) Evaluation of different zeolites in their parent and protonated forms for the catalytic combustion of hexane and benzene. Microporous Mesoporous Mater 83(1–3):292–300
Chiang Y-C, Chiang P-C, Huang C-P (2001) Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 39(4):523–534
Huang Z-H, Kang F, Liang K-M, Hao J (2003) Breakthrough of methyethylketone and benzene vapors in activated carbon fiber beds. J Hazard Mater 98(1–3):107–115
Lillo-Ródenas MA, Fletcher AJ, Thomas KM, Cazorla-Amorós D, Linares-Solano A (2006) Competitive adsorption of a benzene-toluene mixture on activated carbons at low concentration. Carbon 44(8):1455–1463
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2005) Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 43(8):1758–1767
Maldonado-Hódar FJ, Moreno-Castilla C, Carrasco-Marín F, Pérez-Cadenas AF (2007) Reversible toluene adsorption on monolithic carbon aerogels. J Hazard Mater 148(3):548–552
Lin C, Ritter JA (2000) Carbonization and activation of sol–gel derived carbon xerogels. Carbon 38(6):849–861
Denoyel R, Fernandez-Colinas J, Grillet Y, Rouquerol J (1993) Assessment of the surface area and microporosity of activated charcoals from immersion calorimetry and nitrogen adsorption data. Langmuir 9(2):515–518. doi:10.1021/la00026a025
Fairen-Jimenez D, Carrasco-Marin F, Moreno-Castilla C (2008) Inter- and intra-primary-particle structure of monolithic carbon aerogels obtained with varying solvents. Langmuir 24(6):2820–2825. doi:10.1021/la703386q
Carrasco-Marín F, Fairén-Jiménez D, Moreno-Castilla C (2009) Carbon aerogels from gallic acid–resorcinol mixtures as adsorbents of benzene, toluene and xylenes from dry and wet air under dynamic conditions. Carbon 47(2):463–469. doi:10.1016/j.carbon.2008.10.026
Camargo-Trillos D (2011) Modelamiento de adsorción y desorción de compuestos orgánicos volátiles COV’s sobre materiales microporosos para el tratamiento y recuperación de efluentes provenientes de procesos industriales. M.sc., Universidad Nacional de Colombia, Medellín
Rouquerol F, Rouquerol L, Sing K (1999) Adsorption by powders and porous solids. Academic Press, London
Rodriguez-Reinoso Alicante SU (2005) Actived carbon. Universidad de Alicante, Alicante
Neimark AV, Lin Y, Ravikovitch PI, Thommes M (2009) Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 47(7):1617–1628. doi:10.1016/j.carbon.2009.01.050
Gor GY, Thommes M, Cychosz KA, Neimark AV (2012) Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 50(4):1583–1590. doi:10.1016/j.carbon.2011.11.037
Arenas E, Chejne F (2004) The effect of the activating agent and temperature on the porosity development of physically activated coal chars. Carbon 42(12–13):2451–2455. doi:10.1016/j.carbon.2004.04.041
Geankoplis CJ (1993) Transport processes and unit operation, 3rd edn. PTR Prentice-Hall, Englewood Cliffs
Acknowledgments
The authors are grateful to Ph.D Carlos Moreno Castilla for his helpful discussions. Diego Camargo is grateful to the Dirección investigación Universidad Nacional sede Medellin DIME and COLCIENCIAS by supporting this work through Project Number 0201009525 and Doctoral Scholarship program respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chejne, F., Camargo-Trillos, D., Pabón, E. et al. Effect on mass transference phenomena by textural change inside monolithic carbon aerogels. Heat Mass Transfer 51, 1141–1148 (2015). https://doi.org/10.1007/s00231-014-1485-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-014-1485-z