Abstract
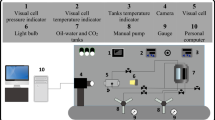
CO2 gas injection is known as one of the most popular enhanced oil recovery techniques for light and medium oil reservoirs, therefore providing an acceptable mass transfer mechanism for CO2–oil systems seems necessary. In this study, interfacial mass transfer coefficient has been evaluated for CO2–normal heptane and CO2–normal hexadecane systems using equilibrium and dynamic interfacial tension data, which have been measured using the pendant drop method. Interface mass transfer coefficient has been calculated as a function of temperature and pressure in the range of 313–393 K and 1.7–8.6 MPa, respectively. The results showed that the interfacial resistance is a parameter that can control the mass transfer process for some CO2–normal alkane systems, and cannot be neglected. Additionally, it was found that interface mass transfer coefficient increased with pressure. However, the variation of this parameter with temperature did not show a clear trend and it was strongly dependent on the variation of diffusivity and solubility of CO2 in the liquid phase.







Similar content being viewed by others
Abbreviations
- r n :
-
Inner radius of needle
- t :
-
Time
- φ :
-
Physical domain
- B im :
-
Boundaries between liquid and impermeable surfaces
- B int :
-
Boundaries between liquid and gas
- c :
-
Gas concentration in liquid phase
- D :
-
Diffusion coefficient
- n r :
-
Direction cosine
- n z :
-
Direction cosine
- k :
-
Interface mass transfer coefficient
- C eq :
-
Equilibrium concentration of gas in liquid
- C :
-
Dimensionless gas concentration
- R :
-
Radial coordinate
- Z :
-
Axial coordinate
- τ :
-
Dimensionless time
- k D :
-
Mass transfer Biot number
- ϴ :
-
Computational domain
- V :
-
Solvent molar volume
- β :
-
Coefficient
- V D :
-
Molar volume of solvent when diffusion approaches zero
- E :
-
Objective function
- IFT exp :
-
Experimental interfacial tension
- IFT cal :
-
Calculated interfacial tension
- T :
-
Absolute temperature
References
Civan F, Rasmussen ML (2001) Accurate measurement of gas diffusivity in oil and brine under reservoir conditions. In: SPE mid continent operations symposium, Oklahoma City, OK
Civan F, Rasmussen ML (2002) Improved measurement of gas diffusivity for miscible gas flooding under nonequilibrium vs. equilibrium conditions. In: SPE/DOE improved oil recovery symposium, Tulsa, OK
Etminan SR, Pooladi-Darvish M, Maini B, Chen ZJ (2010) Mass diffusion parameters in presence of interface resistance in gas–bitumen systems. In: Canadian unconventional resources and international petroleum conference
Saboorian Jooybari H (2012) A novel methodology for simultaneous estimation of gas diffusivity and solubility in bitumens and heavy oils. In: SPE heavy oil conference Canada
Treybal RE (1980) Mass-transfer operations, vol 3. McGraw-Hill, New York
Zhang Y, Hyndman C, Maini B (2000) Measurement of gas diffusivity in heavy oils. J Pet Sci Eng 25(1):37–47
Upreti SR, Mehrotra AK (2000) Experimental measurement of gas diffusivity in bitumen: results for carbon dioxide. Ind Eng Chem Res 39(4):1080–1087
Etminan SR, Maini BB, Chen Z, Hassanzadeh H (2010) Constant–pressure technique for gas diffusivity and solubility measurements in heavy oil and bitumen. Energy Fuels 24(1):533–549
Reamer H, Opfell J, Sage B (1956) Diffusion coefficients in hydrocarbon systems methane-decane-methane in liquid phase-methane-decane-methane in liquid phase. Ind Eng Chem 48(2):275–282
Walas SM (1991) Modeling with differential equations in chemical engineering. Butterworth-Heinemann, Boston, MA
Civan F, Rasmussen M (2006) Determination of gas diffusion and interface–mass transfer coefficients for quiescent reservoir liquids. SPE J 11(1):71–79
Riazi MR (1996) A new method for experimental measurement of diffusion coefficients in reservoir fluids. J Pet Sci Eng 14(3):235–250
Policarpo N (2012) The study of mass transfer between phases in gas and organic drilling fluid mixtures. In: SPE annual technical conference and exhibition
Tharanivasan AK, Yang C, Gu Y (2004) Comparison of three different interface mass transfer models used in the experimental measurement of solvent diffusivity in heavy oil. J Pet Sci Eng 44(3):269–282
Yang C, Gu Y (2005) New experimental method for measuring gas diffusivity in heavy oil by the dynamic pendant drop volume analysis (DPDVA). Ind Eng Chem Res 44(12):4474–4483
Yang C, Gu Y (2005) A novel experimental technique for studying solvent mass transfer and oil swelling effect in the vapour extraction (VAPEX) process. In: Canadian international petroleum conference
Yang D, Gu Y (2008) Determination of diffusion coefficients and interface mass-transfer coefficients of the crude oil−CO2 system by analysis of the dynamic and equilibrium interfacial tensions. Ind Eng Chem Res 47(15):5447–5455
Yang D, Gu Y (2006) A new experimental technique for studying gas mass transfer in the crude oil by analysis of the measured dynamic and equilibrium interfacial tensions. In: SPE annual technical conference and exhibition
Yang D, Tontiwachwuthikul P, Gu Y (2005) Interfacial tensions of the crude oil + reservoir brine + CO2 systems at pressures up to 31 MPA and temperatures of 27 C and 58 C. J Chem Eng Data 50(4):1242–1249
Yang D, Gu Y (2005) Interfacial interactions between crude oil and CO2 under reservoir conditions. Pet Sci Technol 23(9–10):1099–1112
Ayirala SC, Rao DN (2006) A new mechanistic Parachor model to predict dynamic interfacial tension and miscibility in multicomponent hydrocarbon systems. J Colloid Interface Sci 299(1):321–331
Ayirala SC, Rao DN (2006) Solubility, miscibility and their relation to interfacial tension in ternary liquid systems. Fluid Phase Equilib 249(1):82–91
Nobakht M, Moghadam S, Gu Y (2008) Mutual interactions between crude oil and CO2 under different pressures. Fluid Phase Equilib 265(1):94–103
Zolghadr A, Escrochi M, Ayatollahi S (2013) Temperature and composition effect on CO2 miscibility by interfacial tension measurement. J Chem Eng Data 58(5):1168–117525
Zolghadr A, Riazi M, Escrochi M, Ayatollahi S (2013) Investigating the effects of temperature, pressure, and paraffin groups on the n2 miscibility in hydrocarbon liquids using interfacial tension measurement method. Ind Eng Chem Res 52(29):9851–9857
Jůza J (1997) The pendant drop method of surface tension measurement: equation interpolating the shape factor tables for several selected planes. Czech J Phys 47(3):351–357
Drelich J, Fang Ch, White CL (2002) Measurement of interfacial tension in fluid–fluid systems. In: Hubbard AT (ed) Encyclopedia of Surface and Colloid Science. Marcel Dekker, Inc., New York, NY, pp 3152–3166
Yang C, Gu Y (2006) Diffusion coefficients and oil swelling factors of carbon dioxide, methane, ethane, propane, and their mixtures in heavy oil. Fluid Phase Equilib 243(1):64–73
Saatdjian E, Janna W (2001) Transport phenomena: equations and numerical solutions. Appl Mech Rev 54:72
Matthews MA, Rodden JB, Akgerman A (1987) High-temperature diffusion of hydrogen, carbon monoxide, and carbon dioxide in liquid n-heptane, n-dodecane, and n-hexadecane. J Chem Eng Data 32(3):319–322
Saad H, Gulari E (1984) Diffusion of carbon dioxide in heptane. J Phys Chem 88(1):136–139
Perry RH, Green DW, Maloney JO (2008) Perry’s chemical engineers’ handbook, vol 7. McGraw-Hill, New York
Dopierala K, Javadi A, Krägel J, Schano K-H, Kalogianni E, Leser M, Miller R (2011) Dynamic interfacial tensions of dietary oils. Colloids Surf A 382(1):261–265
Nobakht M, Moghadam S, Gu Y (2008) Determination of CO2 minimum miscibility pressure from measured and predicted equilibrium interfacial tensions. Ind Eng Chem Res 47(22):8918–8925
Grogan A, Pinczewski V, Ruskauff G, Orr FM (1988) Diffusion of CO2 at reservoir conditions: models and measurements. SPE Reserv Eng 3(1):93–102
Chaodong Y, Yongan G (2003) A new method for measuring solvent diffusivity in heavy oil by dynamic pendant drop shape analysis (DPDSA). In: SPE annual technical conference and exhibition
Zamanian E, Hemmati M, Beiranvand MS (2012) Determination of gas-diffusion and interface–mass-transfer coefficients in fracture–heavy oil saturated porous matrix system. Nafta 63(11–12):351–358
Acknowledgments
The authors are grateful for the research council of the Shiraz University for financial supports and providing the laboratory and the computational facilities required by the research. Financial supports from Enhanced Oil Recovery (EOR) Center of the College of Engineering are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikkhou, F., Keshavarz, P., Ayatollahi, S. et al. Evaluation of interfacial mass transfer coefficient as a function of temperature and pressure in carbon dioxide/normal alkane systems. Heat Mass Transfer 51, 477–485 (2015). https://doi.org/10.1007/s00231-014-1429-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-014-1429-7