Abstract
Objective: Amisulpride is a substituted benzamide neuroleptic, which binds selectively to dopamine D2 and D3 receptors, mainly in the limbic structures. States of delusion and agitation occur frequently in the population aged more than 65 years, especially in demented patients and this sometimes requires the use of neuroleptics. The objectives of this study were to determine the safety and the pharmacokinetic profile of 50 mg of amisulpride administered orally as a single dose to elderly volunteers.
Methods: Twenty healthy volunteers (10 men and 10 women) aged 65–79 years were included in this open trial. Frequent measurements of blood pressure and heart rate were made and ECG and blood samples were performed up to 72 h after drug intake.
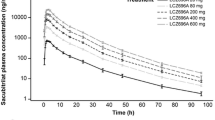
Results: The overall clinical and cardiovascular safety was satisfactory. The mean Cmax of the racemate amisulpride in elderly people was 64.1 ± 6.7 ng · ml−1, and was not different from the value of 56 ± 4.1 ng · ml−1 in young subjects. As with the Cmax, the mean values of t1/2 and AUC in elderly people (15.6 ± 1.3 h and 667 ± 51 ng · ml−1· h, respectively) were not different to values observed in young subject (respectively 11.7 ± 0.5 h and 603 ± 25 ng · ml−1· h).
Conclusions: A single oral dose of amisulpride was well tolerated and showed a similar pharmacokinetic profile in healthy elderly and young subjects. However, these findings should be confirmed after multiple dosing in a larger population in order to establish the lack of need of dosage adjustment in this elderly population.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 October 1997 / Accepted in revised form: 11 March 1998
Rights and permissions
About this article
Cite this article
Hamon-Vilcot, B., Chaufour, S., Deschamps, C. et al. Safety and pharmacokinetics of a single oral dose of amisulpride in healthy elderly volunteers. E J Clin Pharmacol 54, 405–409 (1998). https://doi.org/10.1007/s002280050483
Issue Date:
DOI: https://doi.org/10.1007/s002280050483