Abstract
Objective:
Two different conventional release enalapril maleate tablet formulations were evaluated for their relative bioavailability (Eupressin tablets 10 mg, Biosintética as the test formulation vs Renitec tablets 10 mg Merck Sharp & Dhome, as the reference formulation). A single 20 mg oral dose of each preparation was administered to 18 healthy male adult volunteers and their bioequivalence was assessed by comparing the serum enalaprilat and total enalapril (enalaprilat plus enalapril maleate) concentration-time curves. Angiotensin converting enzyme (ACE) activity was also quantified in each serum sample.
Results:
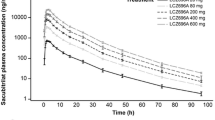
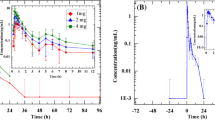
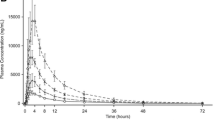
The pharmacokinetic parameters obtained for each formulation were the area under the time-concentration curve from 0 to 24 h (AUC[0–24]), maximum concentration Cmax and the time at which it occurred (tmax). When serum enalaprilat concentration-time curves were employed to assess bioequivalence, the formulations were bioequivalent in the extent but not in the rate of absorption. However, no difference in either the extent or the rate of absorption were observed when serum total enalapril vs time curves were analysed. ACE activity-time curves were similar for both formulations and showed that ACE was 90% inhibited 3–5 h after enalapril administration, and till approximately 50% after 24 h. At that time, circulating enalaprilat and total enalapril levels were less than the tenth of Cmax.
Conclusion:
The results show that complete bioequivalence of the two formulations can be concluded from serum total enalapril concentration data, and that serum ACE activity is not a suitable pharmacodynamic variable for assessing bioequivalence.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 29 May 1995/Accepted in revised form: 30 October 1995
Rights and permissions
About this article
Cite this article
Ribeiro, W., Muscará, M., Martins, A. et al. Bioequivalence study of two enalapril maleate tablet formulations in healthy male volunteers Pharmacokinetic versus pharmacodynamic approach. E J Clin Pharmacol 50, 399–405 (1996). https://doi.org/10.1007/s002280050130
Issue Date:
DOI: https://doi.org/10.1007/s002280050130