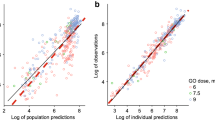
Abstract
Purpose
This study aimed to assess the pharmacokinetics (PK) and pharmacodynamics (PD) of ivosidenib in Chinese patients with relapsed or refractory acute myeloid leukemia (R/R AML) carrying the mIDH1 mutation.
Methods
A bridging study (NCT04176393) was conducted involving 29 Chinese patients who received a daily dose of ivosidenib 500 mg in 28-day cycles. Plasma concentrations of ivosidenib and D-2-hydroxyglutarate (2-HG) were measured before and after treatment. Non-compartmental analysis (NCA) was employed to evaluate the PK, and an established population pharmacokinetic (popPK) model developed from non-Chinese patients was externally validated.
Results
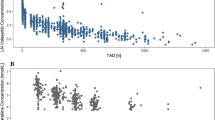
The findings revealed comparable PD effects of ivosidenib in Chinese patients with mIDH1 R/R AML. After adjusting for concomitant drug effects, PK characteristics were similar between Chinese and non-Chinese patients. Furthermore, the popPK model offered additional insights into the possible causes of the apparent ethnic difference in PK exposure.
Conclusion
The study indicates that ivosidenib can be used effectively in Chinese patients, and the observed ethnic differences in PK exposure can be explained by concomitant drug effects. The popPK model contributes to a better understanding and optimization of personalized dosing in Chinese patients with mIDH1 R/R AML.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bullinger L, Dohner K, Dohner H (2017) Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol 35(9):934–946
Guan L et al (2013) The Frequency and clinical significance of IDH1 mutations in Chinese acute myeloid leukemia patients. PLoS ONE 8(12):e83334
Lin J et al (2012) IDH1 and IDH2 mutation analysis in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. Ann Hematol 91(4):519–525
Dang L et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462(7274):739–744
Lu C et al (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483(7390):474–478
Saha SK et al (2014) Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature 513(7516):110–114
Popovici-Muller J et al (2018) Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett 9(4):300–305
Jiang X et al (2021) Population pharmacokinetic and exposure-response analyses of ivosidenib in patients with IDH1-mutant advanced hematologic malignancies. Clin Transl Sci 14(3):942–953
Zhang Y et al (2017) Bioequivalence of generic alendronate sodium tablets (70 mg) to Fosamax((R)) tablets (70 mg) in fasting, healthy volunteers: a randomized, open-label, three-way, reference-replicated crossover study. Drug Des Devel Ther 11:2109–2119
Ling J et al (2015) Population pharmacokinetics of mycophenolic acid and its main glucuronide metabolite: a comparison between healthy Chinese and Caucasian subjects receiving mycophenolate mofetil. Eur J Clin Pharmacol 71(1):95–106
Xu SX et al (2010) Population pharmacokinetics of tapentadol immediate release(IR) in healthy subjects and patients with moderate or severe pain. Clin Pharmacokinet 49(10):671–682
Zhao CY et al (2016) External evaluation of published population pharmacokinetic models of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol 81(5):891–907
Stillemans G et al (2021) Optimal sampling strategies for darunavir and external validation of the underlying population pharmacokinetic model. Eur J Clin Pharmacol 77(4):607–616
Brendel K et al (2006) Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23(9):2036–2049
Brendel K et al (2010) Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn 37(1):49–65
Comets E, Brendel K, Mentre F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90(2):154–166
Fan B et al (2020) Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Invest New Drugs 38(2):433–444
Myrand SP et al (2008) Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther 84(3):347–361
Dai D et al (2019) Effect of itraconazole, food, and ethnic origin on the pharmacokinetics of ivosidenib in healthy subjects. Eur J Clin Pharmacol 75(8):1099–1108
Acknowledgements
We would like to thank the patients who participated in this study and their families, as well as the investigators and research staff and their institutions and study coordinators.
Funding
This work was funded by CStone Pharmaceuticals (Suzhou) Co., Ltd.
Author information
Authors and Affiliations
Contributions
Y. S. and A. N. T. contributed to the conception and methodology of the study. Z. Y., C. P., S. W., and Y. S. participated in the study design; Z. Y.: acquisition, analysis, or interpretation of data, drafting of the manuscript. All authors reviewed, revised, and approved of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yue, Z., Pan, C., Wang, S. et al. Clinical pharmacokinetics and pharmacodynamics of ivosidenib in Chinese patients with relapsed or refractory IDH1-mutated acute myeloid leukemia. Eur J Clin Pharmacol 80, 105–113 (2024). https://doi.org/10.1007/s00228-023-03591-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03591-4