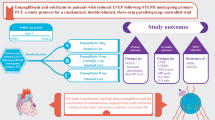
Abstract
Purpose
There is accumulating evidence regarding the potential benefits of empagliflozin in individuals with acute myocardial infarction (MI). Based on the literature, colchicine could also reduce the risk of MI and death in individuals with cardiovascular disease (CVD). However, trials investigating the effects of the combination of empagliflozin with colchicine and high-dose empagliflozin monotherapy in this setting are lacking.
Methods
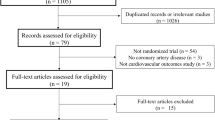
In this trial, 106 non-diabetic participants with reduced left ventricular ejection fraction (LVEF) following recent ST-elevation MI were randomly assigned to empagliflozin 10 mg/day, empagliflozin 10 mg/day plus colchicine 0.5 mg twice daily, or empagliflozin 25 mg/day groups within 72 h after primary percutaneous coronary intervention (PCI). The study’s primary outcomes were the changes in New York Heart Association (NYHA) functional class and high-sensitivity C-reactive protein (hs-CRP) over 12 weeks.
Results
The baseline characteristics of individuals were statistically similar between the study groups. Changes in NYHA functional class over 12 weeks were not significantly different between the study groups. hs-CRP was significantly reduced in all groups (all P < 0.001); however, there was no significant change between the groups over the study period. Changes in tumor necrosis factor-alpha (TNF-α), LVEF, and left ventricular end-diastolic dimension (LVEDD) during the research period did not differ significantly between groups.
Conclusion
This study showed that neither the combination treatment of empagliflozin 10 mg/day with colchicine nor the monotherapy of empagliflozin 25 mg/day was superior to empagliflozin 10 mg/day in terms of changes in clinical, inflammatory, and echocardiographic outcome parameters in patients with recent MI with reduced LVEF over 3 months. Further studies are warranted to confirm the findings.
Trial registration
Clinical trial ID: IRCT20111206008307N39. Registration date: 27 October 2022. https://www.irct.ir/trial/66216



Similar content being viewed by others
Data availability
Data will be made available on request from the corresponding author.
Change history
02 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00228-023-03600-6
References
Tsao CW, Aday AW, Almarzooq ZI et al (2023) Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation 147(8):e93–e621. https://doi.org/10.1161/CIR.0000000000001123
Klaeboe LG, Edvardsen T (2019) Echocardiographic assessment of left ventricular systolic function. J Echocardiogr 17:10–6. https://doi.org/10.21037/jeccm.2019.07.05
Jenča D, Melenovský V, Stehlik J et al (2021) Heart failure after myocardial infarction: incidence and predictors. ESC heart failure 8(1):222–237. https://doi.org/10.1002/ehf2.13144
Udell JA, Jones WS, Petrie MC et al (2022) Sodium glucose cotransporter-2 inhibition for acute myocardial infarction: JACC review topic of the week. J Am Coll Cardiol 79(20):2058–2068. https://doi.org/10.1016/j.jacc.2022.03.353
Heidenreich PA, Bozkurt B, Aguilar D et al (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145(18):e895–e1032. https://doi.org/10.1161/CIR.0000000000001063
von Lewinski D, Kolesnik E, Tripolt NJ et al (2022) Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J 43(41):4421–4432. https://doi.org/10.1093/eurheartj/ehac494
Dayem KA, Younis O, Zarif B, Attia S, AbdelSalam A (2023) Impact of dapagliflozin on cardiac function following anterior myocardial infarction in non-diabetic patients - DACAMI (a randomized controlled clinical trial). Int J Cardiol 379:9–14. https://doi.org/10.1016/j.ijcard.2023.03.002
Hao Z, Zhang Y (2022) Different doses of empagliflozin in patients with heart failure with reduced ejection fraction. Int Heart J 63(5):852–856. https://doi.org/10.1536/ihj.22-151
MacDonald BJ, Turgeon RD (2023) A dose comparison study of empagliflozin in patients with heart failure with preserved ejection fraction. Can J Cardiol S0828–282X(23)00448–8. https://doi.org/10.1016/j.cjca.2023.05.018
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373(22):2117–2128. https://doi.org/10.1056/NEJMoa1504720
Deftereos SG, Beerkens FJ, Shah B et al (2022) Colchicine in cardiovascular disease: in-depth review. Circulation 145(1):61–78. https://doi.org/10.1161/CIRCULATIONAHA.121.056171
Tardif JC, Kouz S, Waters DD et al (2019) Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 381(26):2497–2505. https://doi.org/10.1056/NEJMoa1912388
Deftereos S, Giannopoulos G, Panagopoulou V et al (2014) Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. JACC Heart Fail 2(2):131–137. https://doi.org/10.1016/j.jchf.2013.11.006
Roth ME, Chinn ME, Dunn SP, Bilchick KC, Mazimba S (2022) Association of colchicine use for acute gout with clinical outcomes in acute decompensated heart failure. Clin Cardiol 45(7):733–741. https://doi.org/10.1002/clc.23830
World Medical Association (2013) Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310(20):2191–4. https://doi.org/10.1001/jama.2013.281053
Khiali S, Taban-Sadeghi M, Sarbakhsh P et al (2023) Empagliflozin and colchicine in patients with reduced left ventricular ejection fraction following ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: a study protocol for a randomized, double-blinded, three-arm parallel-group, controlled trial. Trials 24(1):645. https://doi.org/10.1186/s13063-023-07682-6
Ltd SE (2022) Create a blocked randomisation list [Available from: https://www.sealedenvelope.com/simple-randomiser/v1/lists]
Doig GS, Simpson F (2005) Randomization and allocation concealment: a practical guide for researchers. J Crit Care 20(2):187–91; discussion 91–3. https://doi.org/10.1016/j.jcrc.2005.04.005
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Packer M, Anker SD, Butler J et al (2021) Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation 143(4):326–336. https://doi.org/10.1161/CIRCULATIONAHA.120.051783
Fiolet ATL, Silvis MJM, Opstal TSJ et al (2020) Short-term effect of low-dose colchicine on inflammatory biomarkers, lipids, blood count and renal function in chronic coronary artery disease and elevated high-sensitivity C-reactive protein. PLoS ONE 15(8):e0237665. https://doi.org/10.1371/journal.pone.0237665
Shi FH, Li H, Shen L et al (2021) High-dose sodium-glucose co-transporter-2 inhibitors are superior in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 23(9):2125–2136. https://doi.org/10.1111/dom.14452
Wu Q, Liu M, Fang Z et al (2022) Efficacy and safety of empagliflozin at different doses in patients with type 2 diabetes mellitus: a network meta-analysis based on randomized controlled trials. J Clin Pharm Ther 47(3):270–286. https://doi.org/10.1111/jcpt.13521
Carrero JJ, Andersson Franko M, Obergfell A, Gabrielsen A, Jernberg T (2019) hsCRP level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: a healthcare-based study. J Am Heart Assoc 8(11):e012638. https://doi.org/10.1161/JAHA.119.012638
Caraballo C, Desai NR, Mulder H et al (2019) Clinical Implications of the New York Heart Association Classification. J Am Heart Assoc 8(23):e014240. https://doi.org/10.1161/JAHA.119.014240
Zelniker TA, Braunwald E (2019) Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol 75(4):422–434. https://doi.org/10.1016/j.jacc.2019.11.031
Furtado RHM, Bonaca MP, Raz I et al (2019) Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 139(22):2516–2527. https://doi.org/10.1161/CIRCULATIONAHA.119.039996
Packer M, Butler J, Zannad F et al (2021) Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-Preserved trial. Circulation 144(16):1284–1294. https://doi.org/10.1161/CIRCULATIONAHA.121.056824
McMurray JJV, DeMets DL, Inzucchi SE et al (2019) The Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail 21(11):1402–1411. https://doi.org/10.1002/ejhf.1548
Heerspink HJL, Stefansson BV, Chertow GM et al (2020) Rationale and protocol of the Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35(2):274–282. https://doi.org/10.1093/ndt/gfz290
Solomon SD, de Boer RA, DeMets D et al (2021) Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail 23(7):1217–1225. https://doi.org/10.1002/ejhf.2249
Nidorf SM, Fiolet ATL, Mosterd A et al (2020) Colchicine in patients with chronic coronary disease. N Engl J Med 383(19):1838–1847. https://doi.org/10.1002/ejhf.2249
Lodoco (2023) Prescribing information.AGEPHA Pharma FZ LLC [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215727s000lbl.pdf]
Hennessy T, Soh L, Bowman M et al (2019) The low dose colchicine after myocardial infarction (LoDoCo-MI) study: a pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am Heart J 215:62–69. https://doi.org/10.1016/j.ahj.2019.06.003
Andreis A, Imazio M, Avondo S et al (2021) Adverse events of colchicine for cardiovascular diseases: a comprehensive meta-analysis of 14 188 patients from 21 randomized controlled trials. J Cardiovasc Med (Hagerstown) 22(8):637–644. https://doi.org/10.2459/JCM.0000000000001157
Acknowledgements
The authors acknowledge the Cardiovascular Research Center of Shahid Madani Heart Center of Tabriz University of Medical Sciences.
Funding
This work was granted by the Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran, under grant number 69372. The funding party had no role in the study design, data collection, management, analysis, interpretation of data, reporting of the results, and report submission.
Author information
Authors and Affiliations
Contributions
The authors’ eligibility criteria were according to the recommended guideline of the international committee of medical journal editors (ICMJE). TE created the initial concept and designed and supervised the trial. SK, MT, and NK involved in the data collection. PS, TE, and SK involved in the data analysis and interpretation. TE and SK were responsible for drafting the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
The trial was authorized by the research ethics committee of the Tabriz University of Medical Sciences (ethics number: IR.TBZMED.REC.1401.370) and registered in International Clinical Trials Registry Platform with the identifier IRCT20111206008307N39. The study was in accordance with the Declaration of Helsinki and later revisions of ethical principles for medical research.
Consent to participate
Patients provided written informed consent prior to any screening and study-related procedures.
Consent for publication
Patients consented to the publication of the results.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khiali, S., Taban-Sadeghi, M., Sarbakhsh, P. et al. Empagliflozin and colchicine in patients with reduced left ventricular ejection fraction following ST-elevation myocardial infarction: a randomized, double-blinded, three-arm parallel-group, controlled trial. Eur J Clin Pharmacol 80, 93–104 (2024). https://doi.org/10.1007/s00228-023-03582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03582-5