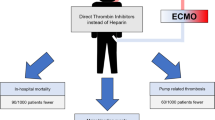
Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is a vital technique for severe respiratory or heart failure patients. Bleeding and thrombotic events are common during ECMO and negatively impact patient outcomes. Unfractionated heparin is the primary anticoagulant, but its adverse effects limit its use, necessitating alternative anticoagulants.
Objective
Review available alternative anticoagulants for adult ECMO patients. Explore potential novel anticoagulants for future ECMO use. Aim to reduce complications (bleeding and thrombosis) and improve safety and efficacy for critically ill ECMO patients.
Methods
Comprehensive literature review of existing and emerging anticoagulants for ECMO.
Results
Identified a range of alternative anticoagulants beyond unfractionated heparin. Evaluated their potential utility in mitigating ECMO-related complications.
Conclusion
Diverse anticoagulant options are available and under investigation for ECMO. These alternatives may enhance patient safety and outcomes during ECMO support. Further research and clinical studies are warranted to determine their effectiveness and safety profiles.




Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in the published studies.
References
Lequier l, Annich G, Al-Ibrahim O, Bembea M, Brodie D (2014) Brogan T.ELSO Anticoagulation guidelines. Ann Arbor MI: Extracorporeal Life Support Organization
Kowalewski M, Fina D, Słomka A et al (2020) COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit Care 24(1):205. https://doi.org/10.1186/s13054-020-02925-3
Supady A, Combes A, Barbaro RP et al (2022) Respiratory indications for ECMO: focus on COVID-19. Intensive Care Med 48(10):1326–1337. https://doi.org/10.1007/s00134-022-06815-w
Badulak J, Antonini MV, Stead CM et al (2021) Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J 67(5):485–495. https://doi.org/10.1097/MAT.0000000000001422
Mansour A, Flecher E, Schmidt M et al (2022) Bleeding and thrombotic events in patients with severe COVID-19 supported with extracorporeal membrane oxygenation: a nationwide cohort study. Intensive Care Med 48(8):1039–1052. https://doi.org/10.1007/s00134-022-06794-y
Jin Y, Zhang Y, Liu J, Zhou Z (2023) Thrombosis and bleeding in patients with COVID-19 requiring extracorporeal membrane oxygenation: a systematic review and meta-analysis. Res Pract Thromb Haemost 7(2):100103. https://doi.org/10.1016/j.rpth.2023.100103
Nunez JI, Gosling AF, O’Gara B et al (2022) Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intens Care Med 48:213–224. https://doi.org/10.1007/s00134-021-06593-x
Chung M, Cabezas FR, Nunez JI et al (2020) Hemocompatibility related adverse events and survival on venoarterial extracorporeal life support: an ELSO registry analysis. JACC Heart Fail 8:892–902. https://doi.org/10.1016/j.jchf.2020.09.004
Panigada M, Cucino A, Spinelli E et al (2020) A randomized controlled trial of antithrombin supplementation during extracorporeal membrane oxygenation. Crit Care Med 48(11):1636–1644. https://doi.org/10.1097/CCM.0000000000004590
Piacente C, Martucci G, Miceli V et al (2020) A narrative review of antithrombin use during veno-venous extracorporeal membrane oxygenation in adults: rationale, current use, effects on anticoagulation, and outcomes. Perfusion 35(6):452–464. https://doi.org/10.1177/0267659120913803
Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P (2013) Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 14(2):e77–e84. https://doi.org/10.1097/PCC.0b013e31827127e4
Rivosecchi RM, Arakelians AR, Ryan J et al (2021) Comparison of anticoagulation strategies in patients requiring venovenous extracorporeal membrane oxygenation: heparin versus bivalirudin. Crit Care Med 49(7):1129–1136. https://doi.org/10.1097/CCM.0000000000004944
Kimmoun A, Oulehri W, Sonneville R et al (2018) Prevalence and outcome of heparin-induced thrombocytopenia diagnosed under veno-arterial extracorporeal membrane oxygenation: a retrospective nationwide study. Intens Care Med 44:1460–1469. https://doi.org/10.1007/s00134-018-5346-y
Singhania N, Bnsal S, Nimmatoori DP et al (2020) Current overview on hypercoagulability in COVID-19. Am J Cardiovasc Drugs 20:393–403. https://doi.org/10.1007/s40256-020-00431-z
Maier CL, Truong AD, Auld SC et al (2020) COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 395:1758–1759. https://doi.org/10.1016/S0140-6736(20)31209-5
Fareed J, Walenga JM, Williamson K, Emanuele RM, Kumar A, Hoppensteadt DA (1985) Studies on the antithrombotic effects and pharmacokinetics of heparin fractions and fragments. Semin Thromb Hemost 11(1):56–74. https://doi.org/10.1055/s-2007-1004360
Fareed J, Hoppensteadt D, Walenga J et al (2003) Pharmacodynamic and pharmacokinetic properties of enoxaparin: implications for clinical practice. Clin Pharmacokinet 42(12):1043–1057. https://doi.org/10.2165/00003088-200342120-00003
Krueger K, Schmutz A, Zieger B, Kalbhenn J (2017) Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients. Artif Organs 41(2):186–192. https://doi.org/10.1111/aor.12737
Gratz J, Pausch A, Schaden E et al (2020) Low molecular weight heparin versus unfractioned heparin for anticoagulation during perioperative extracorporeal membrane oxygenation: a single center experience in 102 lung transplant patients. Artif Organs 44(6):638–646. https://doi.org/10.1111/aor.13642
Wiegele M, Laxar D, Schaden E et al (2022) Subcutaneous enoxaparin for systemic anticoagulation of COVID-19 patients during extracorporeal life support. Front Med (Lausanne) 9:879425. Published 2022 Jul 11. https://doi.org/10.3389/fmed.2022.879425
Circelli A, Bissoni L, Scognamiglio G et al (2023) Anticoagulation on extracorporeal support: an alternative strategy. ASAIO J 69(3):e131. https://doi.org/10.1097/MAT.0000000000001786
Jaspers TCC, Keyany A, Maat B, Meijer K, van den Bemt PMLA, Khorsand N (2022) Therapeutically dosed low molecular weight heparins in renal impairment: a nationwide survey. Eur J Clin Pharmacol 78(9):1469–1479. https://doi.org/10.1007/s00228-022-03344-9
Lequier L, Annich G, Al-Ibrahim O et al (2014) ELSO anticoagulation guidelines. Ann Arbor MI:ELSO 1–17
Knoderer CA, Knoderer HM, Turrentine MW, Kumar M (2006) Lepirudin anticoagulation for heparin-induced thrombocytopenia after cardiac surgery in a pediatric patient. Pharmacotherapy 26(5):709–712. https://doi.org/10.1592/phco.26.5.709
Fitzgerald D, Murphy N (1996) Argatroban: a synthetic thrombin inhibitor of low relative molecular mass. Coron Artery Dis 7(6):455–458
Marchetti M, Barelli S, Gleich T et al (2022) Managing argatroban in heparin-induced thrombocytopenia: a retrospective analysis of 729 treatment days in 32 patients with confirmed heparin-induced thrombocytopenia. Br J Haematol 197(6):766. https://doi.org/10.1111/bjh.18120
Rougé A, Pelen F, Durand M et al (2017) Argatroban for an alternative anticoagulant in HIT during ECMO. J Intens Care 5(1):1–5. https://doi.org/10.1186/s40560-017-0235-y
Menk M, Briem P, Weiss B et al (2017) Efficacy and safety of argatroban in patients with acute respiratory distress syndrome and extracorporeal lung support. Ann Intensive Care 7(1):82. https://doi.org/10.1186/s13613-017-0302-5
Geli J, Capoccia M, Maybauer DM et al (2022) Argatroban anticoagulation for adult extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med 37(4):459–471. https://doi.org/10.1177/0885066621993739
Fisser C, Winkler M, Malfertheiner MV et al (2021) Argatroban versus heparin in patients without heparin-induced thrombocytopenia during venovenous extracorporeal membrane oxygenation: a propensity-score matched study. Crit Care 25:1–10. https://doi.org/10.1186/s13054-021-03581-x
Cho AE, Jerguson K, Peterson J et al (2021) Cost-effectiveness of argatroban versus heparin anticoagulation in adult extracorporeal membrane oxygenation patients. Hosp Pharm 56(4):276–281. https://doi.org/10.1177/0018578719890091
Rajsic S, Breitkopf R, Jadzic D et al (2022) Anticoagulation strategies during extracorporeal membrane oxygenation: a narrative review. J Clin Med 11(17):5147. https://doi.org/10.3390/jcm11175147
Dingman JS, Smith ZR, Coba VE, Peters MA, To L (2020) Argatroban dosing requirements in extracorporeal life support and other critically ill populations. Thromb Res 189:69–76. https://doi.org/10.1016/j.thromres.2020.02.021
Gladwell TD (2002) Bivalirudin: a direct thrombin inhibitor. Clin Therap 24(1):38–58. https://doi.org/10.1016/s0149-2918(02)85004-4
Kam PCA, Kaur N, Thong CL (2005) Direct thrombin inhibitors: pharmacology and clinical relevance. Anaesthesia 60(6):565–574. https://doi.org/10.1111/j.1365-2044.2005.04192.x
Caridi-Scheible M, Nichols K, Gallagher J (2021) Bivalirudin in venovenous extracorporeal membrane oxygenation: moving forward in the real world. Crit Care Med 49(7):1208–1210. https://doi.org/10.1097/CCM.0000000000004964
Koster A, Niedermeyer J, Gummert J, Renner A (2017) Low dose bivalirudin anticoagulation for lung transplantation with extracorporeal membrane oxygenation in a patient with acute heparin-induced thrombocytopenia. Eur J Cardiothorac Surg 51(5):1009–1011
Li DH, Sun MW, Zhang JC, Zhang C, Deng L, Jiang H (2022) Is bivalirudin an alternative anticoagulant for extracorporeal membrane oxygenation (ECMO) patients? A systematic review and meta-analysis. Thromb Res 210:53–62. https://doi.org/10.1016/j.thromres.2021.12.024
Sanfilippo F, La Via L, Murabito P, Pappalardo F, Astuto M (2022) More evidence available for the use of Bivalirudin in patients supported by extracorporeal membrane oxygenation. Thromb Res 211:148–149. https://doi.org/10.1016/j.thromres.2022.02.007
Sanfilippo F, Asmussen S, Maybauer DM et al (2017) Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med 32(5):312–319. https://doi.org/10.1177/0885066616656333
Li MJ, Shi JY, Zhang JH (2022) Bivalirudin vs. heparin in paediatric and adult patients on extracorporeal membrane oxygenation: a meta-analysis. Br J Clin Pharmacol 88(6):2605–2616. https://doi.org/10.1111/bcp.15251
Liu L, Liu F, Tan J, Zhao L (2022) Bivalirudin versus heparin in adult and pediatric patients with extracorporeal membrane oxygenation therapy: a systematic review and meta-analysis. Pharmacol Res 177:106089. https://doi.org/10.1016/j.phrs.2022.106089
Ma M, Liang S, Zhu J et al (2022) The efficacy and safety of bivalirudin versus heparin in the anticoagulation therapy of extracorporeal membrane oxygenation: a systematic review and meta-analysis. Front Pharmacol 13:771563. Published 2022 Apr 14. https://doi.org/10.3389/fphar.2022.771563
Wieruszewski PM, Macielak SA, Nei SD et al (2023) Heparin versus bivalirudin for anticoagulation in adult extracorporeal membrane oxygenation: a systematic review and meta-analysis. ASAIO J 69(2):137–144. https://doi.org/10.1097/MAT.0000000000001808
M’Pembele R, Roth S, Metzger A et al (2022) Evaluation of clinical outcomes in patients treated with heparin or direct thrombin inhibitors during extracorporeal membrane oxygenation: a systematic review and meta-analysis. Thromb J 20(1):42. Published 2022 Jul 28. https://doi.org/10.1186/s12959-022-00401-2
Gu J, Yu H, Lin D (2023) Superiority of bivalirudin over heparin anticoagulation therapy for extracorporeal membrane oxygenation? Too early to draw conclusions. Heliyon 9(2). https://doi.org/10.1016/j.heliyon.2023.e13530
Burstein B, Wieruszewski PM, Zhao YJ, Smischney N (2019) Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation. World J Crit Care Med 8:87–98. https://doi.org/10.5492/wjccm.v8.i6.87
Zheng W, Dai X, Xu B, Tian W, Shi J (2023) Discovery and development of Factor Xa inhibitors (2015–2022). Front Pharmacol 14:1105880. Published 2023 Feb 21. https://doi.org/10.3389/fphar.2023.1105880
Wilde MI, Markham A (1997) Danaparoid: A review of its pharmacology and clinical use in the management of heparin-induced thrombocytopenia. Drugs 54:903–924. https://doi.org/10.2165/00003495-199754060-00008
Phan XT, Nguyen TH, Tran TT et al (2020) Suspected heparin-induced thrombocytopenia in a COVID-19 patient on extracorporeal membrane oxygenation support: a case report. Thromb J 18(1):1–5. https://doi.org/10.1186/s12959-020-00252-9
Parlar AI, Sayar U, Cevirme D, Yuruk MA, Mataraci I (2014) Successful use of fondaparinux in a patient with heparin-induced thrombocytopenia while on extracorporeal membrane oxygenation after mitral valve redo surgery. Int J Artif Organs 37(4):344–347. https://doi.org/10.5301/ijao.5000302
Lu GY, Xu H, Li JH, Chen JK, Ning YG (2023) Safety and outcome of early enteral nutrition in patients receiving extracorporeal membrane oxygenation. Clin Nutr 42(9):1711–1714. https://doi.org/10.1016/j.clnu.2023.07.021
Davis RC 2nd, Durham LA 3rd, Kiraly L, Patel JJ (2021) Safety, tolerability, and outcomes of enteral nutrition in extracorporeal membrane oxygenation. Nutr Clin Pract 36(1):98–104. https://doi.org/10.1002/ncp.10591SundaramS,GikakisN,HackCE,etal
Milling TJ Jr, Middeldorp S, Xu L et al (2023) Final study report of Andexanet Alfa for major bleeding with factor Xa inhibitors. Circulation 147(13):1026–1038. https://doi.org/10.1161/CIRCULATIONAHA.121.057844
Rogers KC, Finks SW (2019) A new option for reversing the anticoagulant effect of factor Xa inhibitors: Andexanet Alfa (ANDEXXA). Am J Med 132(1):38–41. https://doi.org/10.1016/j.amjmed.2018.06.028
Sundaram S, Gikakis N, Hack CE et al (1996) Nafamostat mesilate, a broad spectrum protease inhibitor, modulates platelet, neutrophil and contact activation in simulated extracorporeal circulation. Thromb Haemost 75:76–82
Sanfilippo F, CurròJ M, La Via L et al (2022) Use of nafamostat mesilate for anticoagulation during extracorporeal membrane oxygenation: a systematic review. Artif Organs 46(12):2371–2381. https://doi.org/10.1111/aor.14276
Han SJ, Kim HS, Kim KI et al (2011) Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation. J Korean Med Sci 26(7):945–950. https://doi.org/10.3346/jkms.2011.26.7.945
Lang Y, Zheng Y, Qi B et al (2022) Anticoagulation with nafamostat mesilate during extracorporeal life support. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2022.07.022
Han SJ, Han W, Song HJ et al (2018) Validation of nafamostat mesilate as an anticoagulant in extracorporeal membrane oxygenation: a large-animal experiment. Korean J Thorac Cardiovasc Surg 51:114–121. https://doi.org/10.5090/kjtcs.2018.51.2.114
Han W, San Bok J, Cho HJ et al (2019) Single-center experience of extracorporeal membrane oxygenation mainly anticoagulated with nafamostat mesilate. J Thorac Dis 11:2861–2867. https://doi.org/10.21037/jtd.2019.06.30
Lim JY, Kim JB, Choo SJ, Chung CH, Lee JW, Jung SH (2016) Anticoagulation during extracorporeal membrane oxygenation; nafamostat mesilate versus heparin. Ann Thorac Surg 102(2):534–539. https://doi.org/10.1016/j.athoracsur.2016.01.044
Lee JH, Park JH, Jang JH et al (2022) The role of nafamostat mesilate as a regional anticoagulant during extracorporeal membrane oxygenation. Acute Crit Care 37(2):177–184. https://doi.org/10.4266/acc.2021.01312
Lang Y, Zheng Y, Qi B et al (2022) Anticoagulation with nafamostat mesilate during extracorporeal life support. Intern J Cardiol. https://doi.org/10.1016/j.ijcard.2022.07.022
Hernández-Mitre MP, Tong SYC, Denholm JT et al (2022) Nafamostat mesylate for treatment of COVID-19 in hospitalised patients: a structured, narrative review. Clin Pharmacokinet 61(10):1331–1343. https://doi.org/10.1007/s40262-022-01170-x
Clark JA, Schulman G, Golper TA (2008) Safety and efficacy of regional citrate anticoagulation during 8-hour sustained low-efficiency dialysis. Clin J Am Soc Nephrol 3(3):736–742. https://doi.org/10.2215/CJN.03460807
Shum HP, Kwan AMC, Chan KC et al (2014) The use of regional citrate anticoagulation continuous venovenous hemofiltration in extracorporeal membrane oxygenation. ASAIO J 60(4):413–418. https://doi.org/10.1097/MAT.0000000000000085
Giani M, Scaravilli V, Stefanini F et al (2020) Continuous renal replacement therapy in venovenous extracorporeal membrane oxygenation: a retrospective study on regional citrate anticoagulation. ASAIO J 66:332–338. https://doi.org/10.1097/MAT.0000000000001003
Tiranathanagul K, Jearnsujitwimol O, Susantitaphong P et al (2011) Regional citrate anticoagulation reduces polymorphonuclear cell degranulation in critically ill patients treated with continuous venovenous hemofiltration. Ther Apher Dial 15:556–564. https://doi.org/10.1111/j.1744-9987.2011.00996.x
Akers WS, Oh JJ, Oestreich JH et al (2010) Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol 50:27–35. https://doi.org/10.1177/0091270009344986
De Luca L, Steg PG, Bhatt DL et al (2021) Cangrelor: clinical data, contemporary use, and future perspectives. J Am Heart Assoc 10:e022125. https://doi.org/10.1161/JAHA.121.022125
Vaduganathan M, Qamar A, Badreldin HA et al (2017) Cangrelor use in cardiogenic shock: a single-center real-world experience. JACC Cardiovasc Interv 10(16):1712–1714. https://doi.org/10.1016/j.jcin.2017.07.009
Ciolek AM, Ma KL, Garan AR et al (2020) Use of cangrelor during venoarterial extracorporeal membrane oxygenation following percutaneous coronary intervention. Artif Organs 44:339–340. https://doi.org/10.1111/aor.13563
Katz A, Lewis TC, Arnouk S et al (2021) Clinical use of cangrelor after percutaneous coronary intervention in patients requiring mechanical circulatory support. Ann Pharmacother 55(10):1215–1222. https://doi.org/10.1177/1060028021994621
Baldetti L, Nardelli P, Ajello S et al (2022) Antithrombotic therapy with cangrelor and bivalirudin in venoarterial extracorporeal membrane oxygenation patients undergoing percutaneous coronary intervention: a single-center experience [published online ahead of print, 2022 Dec 12]. ASAIO J. https://doi.org/10.1097/MAT.0000000000001871
Patel JS, Kooda K, Igneri LA (2022) A narrative review of the impact of extracorporeal membrane oxygenation on the pharmacokinetics and pharmacodynamics of critical care therapies [published online ahead of print,2022 Oct 15]. Ann Pharmacother 10600280221126438. https://doi.org/10.1177/10600280221126438
Kreutz RP, Nystrom P, Kreutz Y et al (2013) Inhibition of platelet aggregation by prostaglandin E1 (PGE1) in diabetic patients during therapy with clopidogrel and aspirin. Platelets 24(2):145–150. https://doi.org/10.3109/09537104.2012.661107
Hulshof AM, Vries M, Verhezen P et al (2021) The influence of prostaglandin E1 and use of inhibitor percentage on the correlation between the multiplate and verify now in patients on dual antiplatelet therapy. Platelets 32(4):463–468. https://doi.org/10.1080/09537104.2020.1754378
Goerge T, Ho-Tin-Noe B, Carbo C et al (2008) Inflammation induces hemorrhage in thrombocytopenia. Blood 111:4958–4964. https://doi.org/10.1182/blood-2007-11-123620
Buchtele N, Schörgenhofer C, Schwameis M et al (2022) Add-on prostaglandin E1 in venovenous extracorporeal membrane oxygenation: a randomized, double-blind, placebo-controlled pilot trial. Am J Respir Crit Care Med 206(2):170–177. https://doi.org/10.1164/rccm.202110-2359OC
Kenne E, Nickel KF, Long AT et al (2015) Factor XII: a novel target for safe prevention of thrombosis and inflammation. J Intern Med 278(6):571–585. https://doi.org/10.1111/joim.12430
Kenne E, Renné T (2014) Factor XII: a drug target for safe interference with thrombosis and inflammation. Drug Discov Today 19(9):1459–1464. https://doi.org/10.1016/j.drudis.2014.06.024
Larsson M, Rayzman V, Nolte MW et al (2014) A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med 6(222):222ra17. https://doi.org/10.1126/scitranslmed.3006804
Beck S, Stegner D, Loroch S et al (2021) Generation of a humanized FXII knock-in mouse—a powerful model system to test novel antithrombotic agents. J Thromb Haemost 19(11):2835–2840. https://doi.org/10.1111/jth.15488
Kluge KE, Seljeflot I, Arnesen H, Jensen T, Halvorsen S, Helseth R (2022) Coagulation factors XI and XII as possible targets for anticoagulant therapy. Thromb Res 214:53–62. https://doi.org/10.1016/j.thromres.2022.04.013
Poenou G, Dumitru Dumitru T, Lafaie L, Mismetti V, Heestermans M, Bertoletti L (2022) Factor XI inhibition for the prevention of venous thromboembolism: an update on current evidence and future perspectives. Vasc Health Risk Manag 18:359–373. https://doi.org/10.2147/VHRM.S331614
Koch AW, Schiering N, Melkko S et al (2019) MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood 133(13):1507–1516. https://doi.org/10.1182/blood-2018-10-880849
Yi BA, Freedholm D, Widener N et al (2022) Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa. J Thromb Haemost 20(2):307–315. https://doi.org/10.1111/jth.15577
Perera V, Wang Z, Luettgen J et al (2022) First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci. 15(2):330–342. https://doi.org/10.1111/cts.13148. (published correction appears in Clin Transl Sci. 2022 Apr;15(4):1085)
Lorentz CU, Tucker EI, Verbout NG et al (2021) The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood 138(22):2173–2184. https://doi.org/10.1182/blood.2021011725
Greco A, Laudani C, Spagnolo M et al (2023) Pharmacology and clinical development of factor XI inhibitors. Circulation 147(11):897–913. https://doi.org/10.1161/CIRCULATIONAHA.122.062353
Jiang S, Jia Z, Zheng Y et al (2022) Bifunctional fusion protein targeting both FXIIa and FXIa displays potent anticoagulation effects. Life Sci 309:121021. https://doi.org/10.1016/j.lfs.2022.121021
Afosah DK, Ofori E, Mottamal M et al (2022) Factor IX(a) inhibitors: an updated patent review (2003-present). Expert Opin Ther Pat 32(4):381–400. https://doi.org/10.1080/13543776.2022.2026926
Olson SR, Murphree CR, Zonies D et al (2021) Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J 67(3):290–296. https://doi.org/10.1097/MAT.0000000000001230
Acknowledgements
All authors are grateful to our institution for purchasing some quality journal literature that we can access for free and learn a lot from.
Author information
Authors and Affiliations
Contributions
XLQ and LH conducted the literature search and source document search and wrote the paper. WZS was responsible for reviewing the data and drawing the figures for this article. TS was responsible for the design and full text review of this study; WQ was responsible for the full text review. XLQ and TS were responsible for revising the manuscript, and all authors read and approved the final version. XLQ and LH were responsible for downloading and processing the data in this paper and writing the paper. WZS was responsible for reviewing the data in this paper and drawing the graphs and tables. TS was responsible for the design of this study and reviewing the full text; WQ was responsible for reviewing the full text. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, S., Xu, L., Li, H. et al. Anticoagulants in adult extracorporeal membrane oxygenation: alternatives to standardized anticoagulation with unfractionated heparin. Eur J Clin Pharmacol 79, 1583–1594 (2023). https://doi.org/10.1007/s00228-023-03568-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03568-3