Abstract
Introduction
This study documents imprecision in Japanese reports of adverse events following immunization (AEFI). In doing so, it presents methods to analyze this imprecision.
Methods
These methods include use of unique Japanese data on the validity of certain AEFIs. They also include ways to estimate AEFI rates, which allow comparison of AEFI data between countries. Using US AEFI data for comparison, we show how differences in AEFI reporting systems likely influence AEFI statistics.
Results
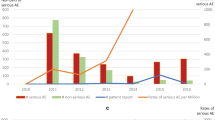
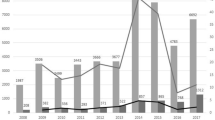
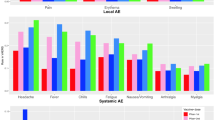
Although our comparisons of AEFI rates are not precise, many of the difference we detected between Japanese and US statistics make sense and reflect differences in the societal and medical perspectives on various vaccines or can be explained by differences in the reporting systems including reporting sources. For example, differences in societal and medical perspective probably underly the extraordinarily high Japanese rates of anaphylaxis and other AEs following HPV immunizations from 2010 to 2016 compared to US rates and to Japanese rates for other vaccines. High US rates of reported Guillain–Barré syndrome following influenza vaccination relative to Japanese rates and to rates for other US vaccines are consistent with data suggesting that the index of suspicion for such reactions could affect AEFI rates. The findings that over half of Japanese anaphylaxis reports for every vaccine are erroneous, and that close to half of “serious” Japanese AEFI cases probably are not serious may be due in part not only to explanations unique to Japan, but also to factors that apply to the USA and other countries. Differences in reporting systems account for a much higher rate of non-serious AEFI reports in the USA compared to Japan. Japanese marketing authorization holders are probably at least as assiduous and timely in their reporting of AEFIs as health care providers, though granular level differences are apparent in reporting by various sources.
Conclusion
The methods we used to analyze the validity of Japanese statistics can be used to analyze the validity of AEFI reports from other countries and aid the harmonization of adverse event reporting systems. Eventually, similar reporting systems might be adapted for drugs and medical devices.
Similar content being viewed by others
Availability of data and material
The findings of this article are based on publicly available data and material.
References
Noguchi Y, Tachi T, Teramachi H (2021) Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform. 22:bbab347. https://doi.org/10.1093/bib/bbab347
Montastruc JL (2022) Pharmacovigilance and drug safety: fair prescribing and clinical research. Therapie 77:261–263. https://doi.org/10.1016/j.therap.2022.03.001
National Institute of Infectious Diseases, Vaccination schedule in Japan, April 1, 2018. https://www.niid.go.jp/niid/images/vaccine/schedule/2018/JP20180401_02.gif. Accessed Mar 2022.
Centers for Disease Control and Prevention, Immunization schedule, February 3 2020. https://www.cdc.gov/vaccines/schedules/index.html. Accessed Mar 2022.
Centers for Disease Control and Prevention, Estimates of influenza vaccination coverage among adults—United States, 2017–18 Flu Season. https://www.cdc.gov/flu/fluvaxview/coverage-1718estimates.htm. Accessed Mar 2022
Kaiser Family Foundation, Population distribution by age, https://www.kff.org/other/state-indicator/distribution-by-age/.Accessed Mar 2022.
Nobuhara H et al (2014) Estimation of influenza vaccination coverage ratio in Japan. Japanese J Public Health 61(7):354–359
Ministry of Health, Labour and Welfare, Outline of the amendment of the Immunization Act, March 30, 2013. http://www.mhlw.go.jp/topics/bcg/tp250330-2.html.
The Law on Securing Quality, Efficacy and safety of products including pharmaceuticals and medical devices, Act No. 145 of 1960. http://www.japaneselawtranslation.go.jp/law/detail/?ft=1&re=01&dn=1&x=35&y=11&co=01&ia=03&ky=%E5%8C%BB%E8%96%AC%E5%93%81%E5%8C%BB%E7%99%82%E6%A9%9F%E5%99%A8&page=2
Code of Federal Regulations Title 21--Food and drugs chapter I--Food and Drug Administration, Department of Health and Human Services subchapter F--Biologics part 600 --biological products: general subpart D--reporting of adverse experiences Sec. 600.80 Postmarketing reporting of adverse experiences. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.80
Tobenkin, An introduction to drug safety surveillance and the FDA adverse event reporting system, April 2018. https://www.fda.gov/files/about%20fda/published/Drug-Safety-Surveillance-and-the-FDA-Adverse-Event-Reporting-System-%28PDF---1.31MB%29.pdf. Accessed Aug 2020
Inokuma Y, Sato Y, Matsuda S (2018) Adverse event reporting patterns of marketing authorization holders, healthcare professionals and patients in Japan: lessons learnt from the human papilloma virus vaccine. Pharmaceutical Medicine 32(2):123–129
Japan Pharmaceutical Manufacturers Association, Protecting patients and its medicines –accumulation and utilization of safety information-, March 2008
Vaccine Adverse Event Reporting System, Table of reportable events following vaccination, March 2017. https://vaers.hhs.gov/docs/VAERS_Table_of_Reportable_Events_Following_Vaccination.pdf. Accessed Aug 2020
National Childhood Vaccine Injury Act of 1986 (42 U.S.C. §§ 300aa-1 to 300aa-34). https://uscode.house.gov/view.xhtml?path=/prelim@title42/chapter6A/subchapter19&edition=prelim
Food and Drug Administration, Guidance for industry postmarketing safety reporting for human drug and biological products including vaccines. March 2001. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Draft-Guidance-for-Industry--Postmarketing-Safety-Reporting-for-Human-Drug-and-Biological-Products-Including-Vaccines.pdf. Accessed Aug 2020
Vaccine Adverse Event Reporting System, VAERS form, February 2020. https://wonder.cdc.gov/wonder/help/vaers/vaers_form_2.0.pdf. Accessed Aug 2020
Vaccine Adverse Event Reporting System, Front page of Vaccine Adverse Event Reporting System (VAERS). https://vaers.hhs.gov/. Accessed Aug 2020
Ministry of Health, Labour and Welfare, Form to report AEFI, 2018. http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/saishin.pdf#search=%27%E5%89%AF%E5%8F%8D%E5%BF%9C+%E6%A7%98%E5%BC%8F%27. Accessed Aug 2020.
Pharmaceuticals and Medical Devices Agency, Pilot and official implementation of patient AE reporting system. https://www.pmda.go.jp/files/000228628.pdf. Accessed Mar 2020
Final report of the committee for investigation of drug-induced hepatitis cases and appropriate regulatory administration to prevent recurrence of drug-induced suffering, April 28, 2010. http://www.mhlw.go.jp/shingi/2010/04/dl/s0428-8a.pdf. Accessed Aug 2020
Ministry of Health, Labour and Welfare, Starting patient adverse reporting system today. https://www.mhlw.go.jp/stf/newpage_04169.html. Accessed Aug 2020
Pharmaceuticals and Medical Devices Agenc, Front page of patient reporting system (PMDA). https://www.pmda.go.jp/safety/reports/patients/0024.html. Accessed Aug 2020
Vaccine Adverse Reactions Review Committee meeting material convened on March 23, 2018. http://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000199232.pdf. Accessed Aug 2020
Vaccine Adverse Event Reporting System, VAERS fact sheet. https://www.cdc.gov/vaccinesafety/00_pdf/09_207294_VAERS_FactSheet.pdf. Accessed Aug 2020
Vaccine Adverse Reactions Review Committee. https://www.mhlw.go.jp/stf/shingi/shingi-yakuji_127869.html, Accessed November 2020
Pharmaceuticals and Medical Devices Agency, Information about adverse events reported to PMDA. https://www.pmda.go.jp/safety/reports/patients/0002.html. Accessed Nov 2020
Vaccine Adverse Event Reporting System. https://vaers.hhs.gov/. Accessed Nov 2020
Gold MS, Gidudu J, Erlewyn-Lajeunesse M, Law B, (2010) Brighton Collaboration Working Group on Anaphylaxis, Can the Brighton Collaboration case definitions be used to improve the quality of adverse event following immunization (AEFI) reporting?: Anaphylaxis as a case study. Vaccine 28(28):4487–4498
Centers for Disease Control and Prevention, ACIP Committee Members, October 2019. https://www.cdc.gov/vaccines/acip/members/index.html. Accessed Aug 2020
Food and Drug Administration, Roster of the Vaccines and Related Biological Products Advisory Committee, April 2020. https://www.fda.gov/advisory-committees/vaccines-and-related-biological-products-advisory-committee/roster-vaccines-and-related-biological-products-advisory-committee. Accessed Aug 2020
Food and Drug Administration, FDA and CDC update on fluzone influenza vaccine and VAERS reports of febrile seizures in children, January 20, 2011. https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/ucm240037.htm. Accessed Aug 2020
Centers for Disease Control and Prevention, ACIP February 2011 summary meeting minutes. https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-feb11.pdf. Accessed Aug 2020
Food and Drug Administration, February 25, 2011: Vaccines and Related Biological Products Advisory Committee meeting transcript. https://wayback.archive-it.org/7993/20161024004521/http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm249303.htm. Accessed Aug 2020
Ministry of Health, Labour and Welfare, Meeting material number 1, Joint meeting of the Vaccine Adverse Reactions Review Committee, 2020. https://www.mhlw.go.jp/content/10601000/000590703.pdf. Accessed Aug 2020.
Inokuma, Y and Kneller, K. Imprecision in adverse event reports following immunization against HPV in Japan and Covid-19 in the US, UK and Japan – and the effects of vaccine hesitancy and government policy. In preparation
Centers for Disease Control and Prevention, Health, United States, 2017. https://www.cdc.gov/nchs/data/hus/hus17.pdf. Accessed Aug 2020
United States Census Bureau, 2014 national population projections datasets. https://www.census.gov/programs-surveys/popproj/data/datasets.html. Accessed Aug 2020
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Clinical safety data management: definitions and standards for expedited reporting E2A. (1994)
Shimabukuro TT et al (2015) Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 33(36):4398–4405
Larson HJ, de Figueiredo A, Zhao XH et al (2016) The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine 12:295–301. https://doi.org/10.1016/j.ebiom.2016.08.042. Accessed Mar 2022
Abbott A (2006) Lyme disease: uphill struggle. Nature 439(7076):524–525
Nakajima H et al (2014) Healthcare professionals’ awareness of adverse effects on eyes caused by anticancer drugs. Iryoyakugaku 40(6):360–368
Japanese Society of Allergology, definition of anaphylaxis. https://www.jsaweb.jp/modules/stwn/index.php?content_id=7. Accessed Mar 2021
Turner PJ, Worm M, Ansotegui IJ et al (2019) Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J 12(10)
Center for Disease Control, Guillain-Barré syndrome and vaccine, August 14 2020. https://www.cdc.gov/vaccinesafety/concerns/guillain-barre-syndrome.html#. Accessed Nov 2020
Center for Disease Control, Estimates of flu vaccination coverage among children — United States, 2017–18 Flu Season, September 2018. https://www.cdc.gov/flu/fluvaxview/coverage-1718estimates-children.htm. Accessed Mar 2021
Tsuzuki S, Schwehm M, Eichner M (2018) Simulation studies to assess the long-term effects of Japan’s change from trivalent to quadrivalent influenza vaccination. Vaccine 36(5):624–630
Kusumi M et al (1995) Epidemiology of inflammatory neurological and inflammatory neuromuscular diseases in Tottori Prefecture. Japan Psychiatry and clinical neurosciences 49(3):169–174
Health Action International, Direct patient reporting in the European Union: a snapshot of reporting systems in seven member states (2015). https://haiweb.org/wp-content/uploads/2015/09/Direct-Patient-Reporting-in-the-EU.pdf. Accessed Aug 2020
Van den Bemt PMLA et al (1999) Adverse drug events in hospitalized patients: a comparison of doctors, nurses and patients as sources of reports. Eur J Clin Pharmacol 55(2):155–158
Gäwert L, Hierse F, Zink A, Strangfeld A (2010) How well do patient reports reflect adverse drug reactions reported by rheumatologists? Agreement of physician-and patient-reported adverse events in patients with rheumatoid arthritis observed in the German biologics register. Rheumatology 50:152–160
Egberts TCG et al (1996) Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. BMJ, 1996 313(7056):530–531
Sekine M et al (2020) Japan’s ongoing crisis on HPV vaccination. Vaccines 8(3):362
Author information
Authors and Affiliations
Contributions
YI: conceptualizing, writing of the original draft. RK: providing guidance, conceptualizing, revising, and editing. All the authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate and for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Inokuma, Y., Kneller, R. Imprecision in vaccine adverse event reporting and a methodological analysis of reporting systems to improve pharmacovigilance and public health. Eur J Clin Pharmacol 79, 989–1002 (2023). https://doi.org/10.1007/s00228-023-03505-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03505-4