Abstract
Objectives
Ramucirumab (RAM) and docetaxel (DOC) are commonly used after first-line therapy for advanced non-small cell lung cancer (NSCLC). Therefore, we aimed to elucidate sequencing strategies of RAM and DOC following prior treatments, including immune checkpoint inhibitor (ICI), cytotoxic agent (CTx) alone, bevacizumab (BEV), and tyrosine kinase inhibitor (TKI).
Methods
We recruited patients with NSCLC who received RAM and DOC and compared the groups with and without prior ICI, CTx alone, BEV, and TKI, respectively. By tumor response to such treatments, the patients were further classified into “complete response (CR) + partial response (PR),” “stable disease,” and “progressive disease” groups, respectively. We compared RAM and DOC efficacy among these groups.
Results
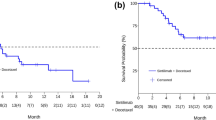
In total, 237 patients were registered. In the group with prior ICI, the objective response rate and disease control rate were significantly higher than those without prior ICI (p = 0.012 and 0.028, respectively), and the median progression-free survival (PFS) was also significantly longer (p = 0.027). There were no significant differences in PFS between the groups with and without CTx alone, BEV, and TKI. Multivariate analysis revealed that prior ICI was an independent factor associated with better PFS. Furthermore, the prior ICI group with CR + PR significantly prolonged PFS compared to the group without prior ICI (p = 0.013).
Conclusion
RAM and DOC may be preferably administered after ICI, rather than after CTx alone, BEV, or TKI, and, furthermore, enhanced if the prior ICI has a favorable tumor response.



Similar content being viewed by others
Data availability
The authors state that they have full control over all primary data and that they agree to allow the journal to review their data if requested.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Ortega-Franco A, Calvo V, Franco F et al (2020) Integrating immune checkpoint inhibitors and targeted therapies in the treatment of early stage non-small cell lung cancer: a narrative review. Transl Lung Cancer Res 9:2656–2673. https://doi.org/10.21037/tlcr-20-546
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/nejmoa1801005
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301. https://doi.org/10.1056/nejmoa1716948
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051. https://doi.org/10.1056/NEJMoa1810865
Shepherd FA, Dancey J, Ramlau R et al (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18:2095–2103. https://doi.org/10.1200/JCO.2000.18.10.2095
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597. https://doi.org/10.1200/JCO.2004.08.163
Kudoh S, Takeda K, Nakagawa K et al (2006) Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 24:3657–3663. https://doi.org/10.1200/JCO.2006.06.1044
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105. https://doi.org/10.1177/1947601911423031
Garon EB, Ciuleanu TE, Arrieta O et al (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384:665–673. https://doi.org/10.1016/S0140-6736(14)60845-X
Matsumoto K, Tamiya A, Matsuda Y et al (2021) Impact of docetaxel plus ramucirumab on metastatic site in previously treated patients with non-small cell lung cancer: a multicenter retrospective study. Transl Lung Cancer Res 10:1642–1652. https://doi.org/10.21037/tlcr-20-1263
Matsumoto K, Tamiya A, Inagaki Y et al (2022) Efficacy and safety of ramucirumab plus docetaxel in older patients with advanced non-small cell lung cancer: a multicenter retrospective cohort study. J Geriatr Oncol 13:207–213. https://doi.org/10.1016/j.jgo.2021.09.004
Harada D, Takata K, Mori S et al (2019) Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer Res 39:4987–4993. https://doi.org/10.21873/anticanres.13688
Tozuka T, Kitazono S, Sakamoto H et al (2020) Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer 144:71–75. https://doi.org/10.1016/j.lungcan.2020.04.021
Kawachi H, Tamiya M, Matsumoto K et al (2022) Efficacy and safety of ramucirumab and docetaxel in previously treated patients with squamous cell lung cancer: a multicenter retrospective cohort study. Invest New Drugs 40:634–642. https://doi.org/10.1007/s10637-022-01214-w
Lee WS, Yang H, Chon HJ, Kim C (2020) Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 52:1475–1485. https://doi.org/10.1038/s12276-020-00500-y
Matsumoto K, Shiroyama T, Kuge T et al (2021) Impact of treatment timing and sequence of immune checkpoint inhibitors and anti-angiogenic agents for advanced non-small cell lung cancer: a systematic review and meta-analysis. Lung Cancer 162:175–184. https://doi.org/10.1016/j.lungcan.2021.11.008
Tseng JS, Yang TY, Chen KC et al (2014) Prior EGFR tyrosine-kinase inhibitor therapy did not influence the efficacy of subsequent pemetrexed plus platinum in advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma. Onco Targets Ther 7:799–805. https://doi.org/10.2147/OTT.S62639
Reck M, Paz-Ares L, Bidoli P et al (2017) Outcomes in patients with aggressive or refractory disease from REVEL: a randomized phase III study of docetaxel with ramucirumab or placebo for second-line treatment of stage IV non-small-cell lung cancer. Lung Cancer 112:181–187. https://doi.org/10.1016/j.lungcan.2017.07.038
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Bhome R, Bullock MD, Al Saihati HA et al (2015) A top-down view of the tumor microenvironment: structure, cells and signaling. Front Cell Dev Biol 3:33. https://doi.org/10.3389/fcell.2015.00033
Nishikawa H, Sakaguchi S (2014) Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 27:1–7. https://doi.org/10.1016/j.coi.2013.12.005
Kumar V, Patel S, Tcyganov E, Gabrilovich DI (2016) The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 37:208–220. https://doi.org/10.1016/j.it.2016.01.004
Pan Y, Yu Y, Wang X, Zhang T (2020) Tumor-associated macrophages in tumor immunity. Front Immunol 11:583084. https://doi.org/10.3389/fimmu.2020.583084
Ma Y, Shurin GV, Peiyuan Z, Shurin MR (2013) Dendritic cells in the cancer microenvironment. J Cancer 4:36–44. https://doi.org/10.7150/jca.5046
Terme M, Pernot S, Marcheteau E et al (2013) VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 73:539–549. https://doi.org/10.1158/0008-5472.CAN-12-2325
Gabrilovich D, Ishida T, Oyama T et al (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92:4150–4166. https://doi.org/10.1182/blood.v92.11.4150
Huinen ZR, Huijbers EJM, van Beijnum JR et al (2021) Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol 18:527–540. https://doi.org/10.1038/s41571-021-00496-y
Voron T, Colussi O, Marcheteau E et al (2015) VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 212:139–148. https://doi.org/10.1084/jem.20140559
Osa A, Uenami T, Koyama S et al (2018) Clinical implications of monitoring nivolumab immunokinetics in non–small cell lung cancer patients. JCI Insight 3. https://doi.org/10.1172/jci.insight.59125
Herbst RS, Garon EB, Kim DW et al (2020) Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol 38:1580–1590. https://doi.org/10.1200/JCO.19.02446
Lee J, Koh J, Kim HK et al (2022) Bevacizumab plus atezolizumab after progression on atezolizumab monotherapy in pretreated patients with NSCLC: an open-label, two-stage, phase 2 trial. J Thorac Oncol 17:900–908. https://doi.org/10.1016/j.jtho.2022.04.001
Reckamp KL, Redman MW, Dragnev KH et al (2022) Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-lung-MAP S1800A. J Clin Oncol 40:2295–2306. https://doi.org/10.1200/JCO.22.00912
Tumeh PC, Harview CL, Yearley JH et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. https://doi.org/10.1038/nature13954
Henning AN, Roychoudhuri R, Restifo NP (2018) Epigenetic control of CD8+ T cell differentiation. Nat Rev Immunol 18:340–356. https://doi.org/10.1038/nri.2017.146
Acknowledgements
We thank all the patients who participated in this study.
Author information
Authors and Affiliations
Contributions
(i) Conception and design: K. Matsumoto and S. Tanizaki; (ii) administrative support: A. Tamiya; (iii) provision of study materials or patients: K. Matsumoto, H. Kawachi, S. Tanizaki, and T. Yanase; (iv) collection and assembly of data: K. Matsumoto; (v) data analysis and interpretation: K. Matsumoto, S. Tanizaki, and A. Tamiya; (vi) manuscript writing: all authors; and (vii) final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Institutional Review Board (IRB) (IRB No. 2020–034) of the National Hospital Organization Kinki-Chuo Chest Medical Center.
Consent to participate
The requirement for consent for participation was waived by the ethics board.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Satoshi Tanizaki and Kinnosuke Matsumoto contributed equally to this manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanizaki, S., Matsumoto, K., Tamiya, A. et al. Sequencing strategies with ramucirumab and docetaxel following prior treatments for advanced non-small cell lung cancer: a multicenter retrospective cohort study. Eur J Clin Pharmacol 79, 503–511 (2023). https://doi.org/10.1007/s00228-023-03452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03452-0