Abstract
Purpose
There is marked heterogeneity in treatment response of atomoxetine in patients with attention deficit/hyperactivity disorder (ADHD), especially for the pediatric population. This review aims to evaluate current evidence to characterize the dose-exposure relationship, establish clinically relevant metrics for systemic exposure to atomoxetine, define a therapeutic exposure range, and to provide a dose-adaptation strategy before implementing personalized dosing for atomoxetine in children with ADHD.
Methods
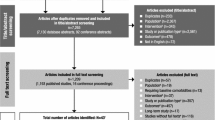
A comprehensive search was performed across electronic databases (PubMed and Embase) covering the period of January 1, 1985 to July 10, 2022, to summarize recent advances in the pharmacokinetics, pharmacogenomics/pharmacogenetics (PGx), therapeutic drug monitoring (TDM), physiologically based pharmacokinetics (PBPK), and population pharmacokinetics (PPK) of atomoxetine in children with ADHD.
Results
Some factors affecting the pharmacokinetics of atomoxetine were summarized, including food, CYP2D6 and CYP2C19 phenotypes, and drug‒drug interactions (DDIs). The association between treatment response and genetic polymorphisms of genes encoding pharmacological targets, such as norepinephrine transporter (NET/SLC6A2) and dopamine β hydroxylase (DBH), was also discussed. Based on well-developed and validated assays for monitoring plasma concentrations of atomoxetine, the therapeutic reference range in pediatric patients with ADHD proposed by several studies was summarized. However, supporting evidence on the relationship between systemic atomoxetine exposure levels and clinical response was far from sufficient.
Conclusion
Personalizing atomoxetine dosage may be even more complex than anticipated thus far, but elucidating the best way to tailor the non-stimulant to a patient’s individual need will be achieved by combining two strategies: detailed research in linking the pharmacokinetics and pharmacodynamics in pediatric patients, and better understanding in nature and causes of ADHD, as well as environmental stressors.

Similar content being viewed by others
Data availability
Data sharing is not applicable.
Abbreviations
- ADHD:
-
Attention deficit/hyperactivity disorder
- AGNP:
-
Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie
- AS:
-
Activity score
- AUC:
-
Area under the time curve
- CD:
-
Conduct disorder
- CL/F:
-
Apparent oral clearance
- C max :
-
Peak concentration
- CPIC:
-
Clinical Pharmacogenetic Implementation Consortium
- CYP2D6:
-
Cytochrome P450 2D6
- DA:
-
Dopamine
- DBH:
-
Dopamine β hydroxylase
- DDIs:
-
Drug–drug interactions
- DPWG:
-
Dutch Pharmacogenetics Working Group
- EM:
-
Extensive metabolizer
- EMs:
-
Extensive metabolizers
- HLMs:
-
Human liver microsomes
- IM:
-
Intermediate metabolizer
- LD:
-
Linkage imbalance
- NAD:
-
Naive average data approach
- NDA:
-
N‐desmethylatomoxetine
- NE:
-
Norepinephrine
- N-desmethyl-4-OH-atomoxetine:
-
N-desmethyl-4-hydroxyatomoxetine
- NET:
-
Norepinephrine transporter
- NET/SLC6A2:
-
Norepinephrine transporter
- NONMEM:
-
Nonlinear mixed-effects modeling approach
- NPD:
-
Naive pooled data analysis
- ODD:
-
Oppositional defiant disorder
- PBPK:
-
Physiologically based pharmacokinetics
- PFC:
-
Prefrontal cortex
- PGx:
-
Pharmacogenomics
- PM:
-
Poor metabolizers
- PMs:
-
Poor metabolizers
- PPK:
-
Population pharmacokinetics
- TDM:
-
Therapeutic drug monitoring
- T max :
-
Time to maximum plasma concentration
- t 1/2 :
-
Half-life
- UM:
-
Ultrarapid metabolizer
- 2-CH2OH-atomoxetine:
-
2‐Hydroxymethylatomoxetine
- 4-OH-atomoxetine:
-
4-Hydroxyatomoxetine
- 4-OH-atomoxetine-O-glucuronide:
-
4-Hydroxyatomoxetine-O-glucuronide
References
Thomas R, Sanders S, Doust J, Beller E, Glasziou P (2015) Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135(4):e994-1001
Pozzi M, Carnovale C, Peeters G, Gentili M, Antoniazzi S, Radice S et al (2018) Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord 238:161–178
Clemow D, Bushe C, Mancini M, Ossipov M, Upadhyaya H (2017) A review of the efficacy of atomoxetine in the treatment of attention-deficit hyperactivity disorder in children and adult patients with common comorbidities. Neuropsychiatr Dis Treat 13:357–371
Wolraich M, Hagan J, Allan C, Chan E, Davison D, Earls M et al (2019) Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 144(4):e20192528
Dalsgaard S, Leckman J, Mortensen P, Nielsen H, Simonsen M (2015) Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry 2(8):702–709
Pearson D, Santos C, Aman M, Arnold L, Casat C, Mansour R et al (2013) Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. J Child Adolesc Psychopharmacol 23(5):337–351
Barner J, Khoza S, Oladapo A (2011) ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin 27 Suppl 2:13–22
Mechler K, Banaschewski T, Hohmann S, Häge A (2021) Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther 230:107940
Elsayed N, Yamamoto K, Froehlich T (2020) Genetic influence on efficacy of pharmacotherapy for pediatric attention-deficit/hyperactivity disorder: overview and current status of research. CNS Drugs 34(4):389–414
Mamiya P, Arnett A, Stein M (2021) Precision medicine care in ADHD: the case for neural excitation and inhibition. Brain Sci 11(1):91
Shaker N, Osama Y, Barakat D, Abdelgawad A, Abdel Aziz K, Aly E-G (2021) Atomoxetine in attention-deficit/hyperactivity disorder in children with and without comorbid mood disorders. J Child Adolesc Psychopharmacol 31(5):332–341
Hutchison S, Ghuman J, Ghuman H, Karpov I, Schuster J (2016) Efficacy of atomoxetine in the treatment of attention-deficit hyperactivity disorder in patients with common comorbidities in children, adolescents and adults: a review. Thera Adv Psychopharmacol 6(5):317–334
Cutler A, Mattingly G, Jain R, O’Neal W (2022) Current and future nonstimulants in the treatment of pediatric ADHD: monoamine reuptake inhibitors, receptor modulators, and multimodal agents. CNS Spectr 27(2):199–207
Brown J, Abdel-Rahman S, van Haandel L, Gaedigk A, Lin Y, Leeder J (2016) Single dose, CYP2D6 genotype-stratified pharmacokinetic study of atomoxetine in children with ADHD. Clin Pharmacol Ther 99(6):642–650
Bolea-Alamañac B, Nutt D, Adamou M, Asherson P, Bazire S, Coghill D et al (2014) Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the British Association for Psychopharmacology. J Psychopharmacol (Oxford, England) 28(3):179–203
Tsujii N, Usami M, Naya N, Tsuji T, Mishima H, Horie J et al (2021) Efficacy and safety of medication for attention-deficit hyperactivity disorder in children and adolescents with common comorbidities: a systematic review. Neurol Ther 10(2):499–522
Childress AC (2016) A critical appraisal of atomoxetine in the management of ADHD. Ther Clin Risk Manag 12:27–39
Pliszka S (2007) Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46(7):894–921
Schwartz S, Correll C (2014) Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry 53(2):174–187
Newcorn J, Sutton V, Weiss M, Sumner C (2009) Clinical responses to atomoxetine in attention-deficit/hyperactivity disorder: the Integrated Data Exploratory Analysis (IDEA) study. J Am Acad Child Adolesc Psychiatry 48(5):511–518
Sugimoto A, Suzuki Y, Orime N, Hayashi T, Yoshinaga K, Egawa J et al (2021) The lowest effective plasma concentration of atomoxetine in pediatric patients with attention deficit/hyperactivity disorder: a non-randomized prospective interventional study. Medicine 100(27):e26552
Treuer T, Méndez L, Montgomery W, Wu S (2016) Factors affecting treatment adherence to atomoxetine in ADHD: a systematic review. Neuropsychiatr Dis Treat 12:1061–1083
Hiemke C, Bergemann N, Clement H, Conca A, Deckert J, Domschke K et al (2018) Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry 51(1–02):9–62
Ruppert K, Geffert C, Clement H, Bachmann C, Haberhausen M, Schulz E et al (2022) Therapeutic drug monitoring of atomoxetine in children and adolescents with attention-deficit/ hyperactivity disorder: a naturalistic study. J Neural Transm 129(7):945–959
ter Laak M, Temmink A, Koeken A, van’t Veer N, van Hattum P, Cobbaert C (2010) Recognition of impaired atomoxetine metabolism because of low CYP2D6 activity. Pediatr Neurol 43(3):159–162
Bengtsson F (2004) Therapeutic drug monitoring of psychotropic drugs. TDM “nouveau.” Ther Drug Monit 26(2):145–151
Hiemke C (2008) Therapeutic drug monitoring in neuropsychopharmacology: does it hold its promises? Eur Arch Psychiatry Clin Neurosci 21–27
Jaquenoud Sirot E, van der Velden J, Rentsch K, Eap C, Baumann P (2006) Therapeutic drug monitoring and pharmacogenetic tests as tools in pharmacovigilance. Drug Saf 29(9):735–768
Jang S, Yan Z, Lazor J (2016) Therapeutic drug monitoring: a patient management tool for precision medicine. Clin Pharmacol Ther 99(2):148–150
Crews K, Hicks J, Pui C, Relling M, Evans W (2012) Pharmacogenomics and individualized medicine: translating science into practice. Clin Pharmacol Ther 92(4):467–475
Brown JT, Bishop JR, Sangkuhl K, Nurmi EL, Mueller DJ, Dinh JC et al (2019) Clinical pharmacogenetics implementation consortium guideline for cytochrome P450 (CYP)2D6 genotype and atomoxetine therapy. Clin Pharmacol Ther 106(1):94–102
Jung E, Lee Y, Kim D, Kang P, Lim C, Cho C et al (2020) Effects of paroxetine on the pharmacokinetics of atomoxetine and its metabolites in different CYP2D6 genotypes. Arch Pharmacal Res 43(12):1356–1363
Sauer J, Ring B, Witcher J (2005) Clinical pharmacokinetics of atomoxetine. Clin Pharmacokinet 44(6):571–590
Yu G, Li G, Markowitz J (2016) Atomoxetine: a review of its pharmacokinetics and pharmacogenomics relative to drug disposition. J Child Adolesc Psychopharmacol 26(4):314–326
Papaseit E, Marchei E, Farré M, Garcia-Algar O, Pacifici R, Pichini S (2013) Concentrations of atomoxetine and its metabolites in plasma and oral fluid from paediatric patients with attention deficit/hyperactivity disorder. Drug Test Anal 5(6):446–452
Witcher J, Long A, Smith B, Sauer J, Heilgenstein J, Wilens T et al (2003) Atomoxetine pharmacokinetics in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 13(1):53–63
Caballero J, Nahata M (2003) Atomoxetine hydrochloride for the treatment of attention-deficit/hyperactivity disorder. Clin Ther 25(12):3065–3083
Sauer J, Ponsler G, Mattiuz E, Long A, Witcher J, Thomasson H et al (2003) Disposition and metabolic fate of atomoxetine hydrochloride: the role of CYP2D6 in human disposition and metabolism. Drug Metab Dispos 31(1):98–107
Christman A, Fermo J, Markowitz J (2004) Atomoxetine, a novel treatment for attention-deficit-hyperactivity disorder. Pharmacotherapy 24(8):1020–1036
Michelson D, Read H, Ruff D, Witcher J, Zhang S, McCracken J (2007) CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry 46(2):242–251
Loghin C, Haber H, Beasley C, Kothare P, Kauffman L, April J et al (2013) Effects of atomoxetine on the QT interval in healthy CYP2D6 poor metabolizers. Br J Clin Pharmacol 75(2):538–549
Ramsey L, Brown J, Vear S, Bishop J, Van Driest S (2020) Gene-based dose optimization in children. Annu Rev Pharmacol Toxicol 60:311–331
Ring B, Gillespie J, Eckstein J, Wrighton S (2002) Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos 30(3):319–323
Trzepacz P, Williams D, Feldman P, Wrishko R, Witcher J, Buitelaar J (2008) CYP2D6 metabolizer status and atomoxetine dosing in children and adolescents with ADHD. Eur Neuropsychopharmacol 18(2):79–86
Dinh J, Pearce R, Van Haandel L, Gaedigk A, Leeder J (2016) Characterization of atomoxetine biotransformation and implications for development of PBPK models for dose individualization in children. Drug Metab Dispos 44(7):1070–1079
Protti M, Mandrioli R, Marasca C, Cavalli A, Serretti A, Mercolini L (2020) New-generation, non-SSRI antidepressants: drug-drug interactions and therapeutic drug monitoring. Part 2: NaSSAs, NRIs, SNDRIs, MASSAs, NDRIs, and others. Med Res Rev 40(5):1794–1832
Kim S, Byeon J, Kim Y, Lee C, Lee Y, Jang C et al (2018) Physiologically based pharmacokinetic modelling of atomoxetine with regard to CYP2D6 genotypes. Sci Rep 8(1):12405
You Y, Wang X, Ma K, Li J, Peng Y, Zheng J (2021) Metabolic activation of atomoxetine mediated by cytochrome P450 2D6. Chem Res Toxicol
Mattiuz E, Ponsler G, Barbuch R, Wood P, Mullen J, Shugert R et al (2003) Disposition and metabolic fate of atomoxetine hydrochloride: pharmacokinetics, metabolism, and excretion in the Fischer 344 rat and beagle dog. Drug Metab Dispos 31(1):88–97
Spiller H, Hays H, Aleguas A (2013) Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs 27(7):531–543
Corponi F, Fabbri C, Serretti A (2019) Pharmacogenetics and depression: a critical perspective. Psychiatry Investig 16(9):645–653
Gaedigk A, Jaime L, Bertino J, Bérard A, Pratt V, Bradfordand L et al (2010) Identification of novel CYP2D7-2D6 hybrids: non-functional and functional variants. Front Pharmacol 1:121
Alali M, Ismail Al-Khalil W, Rijjal S, Al-Salhi L, Saifo M, Youssef L (2022) Frequencies of CYP2D6 genetic polymorphisms in Arab populations. Hum Genomics 16(1):6
Crews K, Gaedigk A, Dunnenberger H, Leeder J, Klein T, Caudle K et al (2014) Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther 95(4):376–382
Gaedigk A, Simon S, Pearce R, Bradford L, Kennedy M, Leeder J (2008) The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83(2):234–242
Caudle K, Sangkuhl K, Whirl-Carrillo M, Swen J, Haidar C, Klein T et al (2020) Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the clinical pharmacogenetics implementation consortium and dutch pharmacogenetics working group. Clin Transl Sci 13(1):116–124
Dorji P, Tshering G, Na-Bangchang K (2019) CYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in South-East and East Asian populations: a systematic review. J Clin Pharm Ther 44(4):508–524
Swen J, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee A, Mulder H et al (2011) Pharmacogenetics: from bench to byte–an update of guidelines. Clin Pharmacol Ther 89(5):662–673
Bradford L (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 3(2):229–243
Gaedigk A (2013) Complexities of CYP2D6 gene analysis and interpretation. Int Rev Psychiatry (Abingdon, England) 25(5):534–553
Brown J, Bishop J (2015) Atomoxetine pharmacogenetics: associations with pharmacokinetics, treatment response and tolerability. Pharmacogenomics 16(13):1513–1520
Lan B, Ma F, Zhai X, Li Q, Chen S, Wang J et al (2018) The relationship between the CYP2D6 polymorphisms and tamoxifen efficacy in adjuvant endocrine therapy of breast cancer patients in Chinese Han population. Int J Cancer 143(1):184–189
Byeon J, Kim Y, Lee C, Kim S, Chae W, Jung E et al (2018) CYP2D6 allele frequencies in Korean population, comparison with East Asian, Caucasian and African populations, and the comparison of metabolic activity of CYP2D6 genotypes. Arch Pharmacal Res 41(9):921–930
Mbavha B, Kanji C, Stadler N, Stingl J, Stanglmair A, Scholl C et al (2022) Population genetic polymorphisms of pharmacogenes in Zimbabwe, a potential guide for the safe and efficacious use of medicines in people of African ancestry. Pharmacogenet Genomics 32(5):173–182
Furman K, Grimm D, Mueller T, Holley-Shanks R, Bertz R, Williams L et al (2004) Impact of CYP2D6 intermediate metabolizer alleles on single-dose desipramine pharmacokinetics. Pharmacogenetics 14(5):279–284
LLerena A, Naranjo M, Rodrigues-Soares F, Penas-LLedó E, Fariñas H, Tarazona-Santos E (2014) Interethnic variability of CYP2D6 alleles and of predicted and measured metabolic phenotypes across world populations. Expert Opin Drug Metab Toxicol 10(11):1569–1583
Zhou W, Jiang Y, Xu Y, Wang Y, Ma X, Zhou L et al (2022) Comparison of adverse drug reactions between tamoxifen and toremifene in breast cancer patients with different CYP2D6 genotypes: a propensity-score matched cohort study. Int J Cancer 150(10):1664–1676
Liang B, Zhan Y, Wang Y, Gu E, Dai D, Cai J et al (2016) Effect of 24 Cytochrome P450 2D6 variants found in the Chinese population on atomoxetine metabolism in vitro. Pharmacology 97:78–83
Cai W, Chen B, Zhang W (2007) Frequency of CYP2D6*10 and *14 alleles and their influence on the metabolic activity of CYP2D6 in a healthy Chinese population. Clin Pharmacol Ther 81(1):95–98
Qiu F, Liu S, Miao P, Zeng J, Zhu L, Zhao T et al (2016) Effects of the Chinese herbal formula “Zuojin Pill” on the pharmacokinetics of dextromethorphan in healthy Chinese volunteers with CYP2D6*10 genotype. Eur J Clin Pharmacol 72(6):689–695
Lan B, Ma F, Chen S, Wang W, Li Q, Fan Y et al (2018) Toremifene, rather than tamoxifen, might be a better option for the adjuvant endocrine therapy in CYP2D6*10T/T genotype breast cancer patients in China. Int J Cancer 143(10):2499–2504
Cui Y, Teng C, Pan A, Yuen E, Yeo K, Zhou Y et al (2007) Atomoxetine pharmacokinetics in healthy Chinese subjects and effect of the CYP2D6*10 allele. Br J Clin Pharmacol 64(4):445–449
Matsui A, Azuma J, Witcher J, Long A, Sauer J, Smith B et al (2012) Pharmacokinetics, safety, and tolerability of atomoxetine and effect of CYP2D6*10/*10 genotype in healthy Japanese men. J Clin Pharmacol 52(3):388–403
Byeon J, Kim Y, Na H, Jang J, Kim S, Lee Y et al (2015) Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharmacal Res 38(11):2083–2091
Teh L, Bertilsson L (2012) Pharmacogenomics of CYP2D6: molecular genetics, interethnic differences and clinical importance. Drug Metab Pharmacokinet 27(1):55–67
Ingelman-Sundberg M, Sim S, Gomez A, Rodriguez-Antona C (2007) Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 116(3):496–526
Llerena A, Dorado P, Peñas-Lledó E (2009) Pharmacogenetics of debrisoquine and its use as a marker for CYP2D6 hydroxylation capacity. Pharmacogenomics 10(1):17–28
Chiba K, Kato M, Ito T, Suwa T, Sugiyama Y (2012) Inter-individual variability of in vivo CYP2D6 activity in different genotypes. Drug Metab Pharmacokinet 27(4):405–413
Witcher J, Kurtz D, Heathman M, Sauer J, Smith B (2004) Population pharmacokinetic analysis of atomoxetine in pediatric patients. Clin Pharmacol Ther 75(2):P46–P46
Garnock-Jones K, Keating G (2009) Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs 11(3):203–226
Fijal B, Guo Y, Li S, Ahl J, Goto T, Tanaka Y et al (2015) CYP2D6 predicted metabolizer status and safety in adult patients with attention-deficit hyperactivity disorder participating in a large placebo-controlled atomoxetine maintenance of response clinical trial. J Clin Pharmacol 55(10):1167–1174
Choi C, Bae J, Lee Y, Lee H, Jang C, Lee S (2014) Effects of CYP2C19 genetic polymorphisms on atomoxetine pharmacokinetics. J Clin Psychopharmacol 34(1):139–142
Demirci E, Sener E, Gul M, Onal M, Dal F (2022) A view of response and resistance to atomoxetine treatment in children with ADHD: effects of CYP2C19 polymorphisms and BDNF levels. Eur J Clin Pharmacol 78(7):1095–1104
Zhou S, Liu J, Chowbay B (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41(2):89–295
Desta Z, Zhao X, Shin J, Flockhart D (2002) Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41(12):913–958
Strom CM, Goos D, Crossley B, Zhang K, Sun W (2012) Testing for variants in CYP2C19: population frequencies and testing experience in a clinical laboratory. Genet Med 14(1):95–100
Scott S, Sangkuhl K, Gardner E, Stein C, Hulot J, Johnson J et al (2011) Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450–2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther 90(2):328–332
Martis S, Peter I, Hulot J, Kornreich R, Desnick R, Scott S (2013) Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenomics J 13(4):369–377
Spina E, de Leon J (2015) Clinical applications of CYP genotyping in psychiatry. J Neural Transm 122(1):5–28
Sim S, Risinger C, Dahl M, Aklillu E, Christensen M, Bertilsson L et al (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79(1):103–113
Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J (2010) Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol 69(3):222–230
Clemow D, Bushe C (2015) Atomoxetine in patients with ADHD: a clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients. J Psychopharmacol (Oxford, England) 29(12):1221–1230
Camporeale A, Porsdal V, De Bruyckere K, Tanaka Y, Upadhyaya H, Deix C et al (2015) Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol (Oxford, England) 29(1):3–14
Bymaster F, Katner J, Nelson D, Hemrick-Luecke S, Threlkeld P, Heiligenstein J et al (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27(5):699–711
Callahan P, Plagenhoef M, Blake D, Terry A (2019) Atomoxetine improves memory and other components of executive function in young-adult rats and aged rhesus monkeys. Neuropharmacology 155:65–75
Kratochvil C, Vaughan B, Daughton J, Mayfield-Jorgensen M, Burke W (2004) Atomoxetine in the treatment of attention deficit hyperactivity disorder. Expert Rev Neurother 4(4):601–611
Easton N, Steward C, Marshall F, Fone K, Marsden C (2007) Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology 52(2):405–414
Arnsten A (2011) Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiat 69(12):e89-99
Savill N, Buitelaar J, Anand E, Day K, Treuer T, Upadhyaya H et al (2015) The efficacy of atomoxetine for the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a comprehensive review of over a decade of clinical research. CNS Drugs 29(2):131–151
Ramoz N, Boni C, Downing A, Close S, Peters S, Prokop A et al (2009) A haplotype of the norepinephrine transporter (Net) gene Slc6a2 is associated with clinical response to atomoxetine in attention-deficit hyperactivity disorder (ADHD). Neuropsychopharmacology 34(9):2135–2142
Yang L, Qian Q, Liu L, Li H, Faraone S, Wang Y (2013) Adrenergic neurotransmitter system transporter and receptor genes associated with atomoxetine response in attention-deficit hyperactivity disorder children. J Neural Transm 120(7):1127–1133
Ray A, Maitra S, Chatterjee M, Ghosh P, Karmakar A, Sinha S et al (2017) Dimorphic association of dopaminergic transporter gene variants with treatment outcome: pilot study in Indian ADHD probands. Meta Gene. 11(C):64–69
Gul M, Sener E, Onal M, Demirci E (2021) Role of the norepinephrine transporter polymorphisms in atomoxetine treatment: from response to side effects in children with ADHD. J Psychopharmacol 36(6):715-722
Fang Y, Ji N, Cao Q, Su Y, Chen M, Wang Y et al (2015) Variants of dopamine beta hydroxylase gene moderate atomoxetine response in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 25(8):625–632
Rybak Y, McNeely H, Mackenzie B, Jain U, Levitan R (2007) Seasonality and circadian preference in adult attention-deficit/hyperactivity disorder: clinical and neuropsychological correlates. Compr Psychiatry 48(6):562–571
Faltraco F, Palm D, Uzoni A, Simon F, Thome J (2021) Atomoxetine and circadian gene expression in human dermal fibroblasts from study participants with a diagnosis of attention-deficit hyperactivity disorder. J Neural Transm 128(7):1121–1133
Hvolby A (2015) Associations of sleep disturbance with ADHD: implications for treatment. Atten Defic Hyperact Disord 7(1):1–18
Korman M, Palm D, Uzoni A, Faltraco F, Tucha O, Thome J et al (2020) ADHD 24/7: Circadian clock genes, chronotherapy and sleep/wake cycle insufficiencies in ADHD. World J Biol Psychiatry 21(3):156–171
Coogan A, Schenk M, Palm D, Uzoni A, Grube J, Tsang A et al (2019) Impact of adult attention deficit hyperactivity disorder and medication status on sleep/wake behavior and molecular circadian rhythms. Neuropsychopharmacology 44(7):1198–1206
Wille S, Cooreman S, Neels H, Lambert W (2008) Relevant issues in the monitoring and the toxicology of antidepressants. Crit Rev Clin Lab Sci 45(1):25–89
Mullen J, Shugert R, Ponsler G, Li Q, Sundaram B, Coales H et al (2005) Simultaneous quantification of atomoxetine as well as its primary oxidative and O-glucuronide metabolites in human plasma and urine using liquid chromatography tandem mass spectrometry (LC/MS/MS). J Pharm Biomed Anal 38(4):720–733
Choong E, Rudaz S, Kottelat A, Guillarme D, Veuthey J, Eap C (2009) Therapeutic drug monitoring of seven psychotropic drugs and four metabolites in human plasma by HPLC-MS. J Pharm Biomed Anal 50(5):1000–1008
Patel C, Patel M, Rani S, Nivsarkar M, Padh H (2007) A new high performance liquid chromatographic method for quantification of atomoxetine in human plasma and its application for pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci 850:356–360
Xia Y, Guo H, Hu Y, Long J, Chen J, Chen F et al (2021) Determination of atomoxetine levels in human plasma using LC-MS/MS and clinical application to Chinese children with ADHD based on CPIC guidelines. Anal Methods 13(21):2434–2441
Schoretsanitis G, Paulzen M, Unterecker S, Schwarz M, Conca A, Zernig G et al (2018) TDM in psychiatry and neurology: a comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians. World J Biol Psychiatry 19(3):162–174
Kiang T, Sherwin C, Spigarelli M, Ensom M (2012) Fundamentals of population pharmacokinetic modelling : modelling and software. Clin Pharmacokinet 51(8):515–525
Shi C, Xiao Y, Mao Y, Wu J, Lin N (2019) Voriconazole: A review of population pharmacokinetic analyses. Clin Pharmacokinet 58(6):687–703
Huang W, Nakano M, Sager J, Ragueneau-Majlessi I, Isoherranen N (2017) Physiologically based pharmacokinetic model of the CYP2D6 probe atomoxetine: extrapolation to special populations and drug-drug interactions. Drug Metab Dispos 45(11):1156–1165
Notsu Y, Shimizu M, Sasaki T, Nakano A, Ota M, Yoshida S et al (2020) Simple pharmacokinetic models accounting for drug monitoring results of atomoxetine and its 4-hydroxylated metabolites in Japanese pediatric patients genotyped for cytochrome P450 2D6. Drug Metab Pharmacokinet 35(2):191–200
Barrett J, Della Casa Alberighi O, Läer S, Meibohm B (2012) Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther 92(1):40–49
Abdel-Rahman S, Amidon G, Kaul A, Lukacova V, Vinks A, Knipp G (2012) Summary of the national institute of child health and human development-best pharmaceuticals for children act pediatric formulation initiatives workshop-pediatric biopharmaceutics classification system working group. Clin Ther 34(11):S11-24
Edginton A, Schmitt W, Willmann S (2006) Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet 45(10):1013–1034
Jensen CM, Steinhausen HC (2015) Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. ADHD Atten Defic Hyperact Disord 7(1):27–38
Dell’Agnello G, Zuddas A, Masi G, Curatolo P, Besana D, Rossi A (2009) Use of atomoxetine in patients with attention-deficit hyperactivity disorder and co-morbid conditions. CNS Drugs 23(9):739–753
Steinhausen H, Nøvik T, Baldursson G, Curatolo P, Lorenzo M, Rodrigues Pereira R et al (2006) Co-existing psychiatric problems in ADHD in the ADORE cohort. Eur Child Adolesc Psychiatry 18(3):194–196
Newcorn J, Spencer T, Biederman J, Milton D, Michelson D (2005) Atomoxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry 44(3):240–248
Kratochvil C, Michelson D, Newcorn J, Weiss M, Busner J, Moore R et al (2007) High-dose atomoxetine treatment of ADHD in youths with limited response to standard doses. J Am Acad Child Adolesc Psychiatry 46(9):1128–1137
Gadde K, Yonish G, Wagner H, Foust M, Allison D (2006) Atomoxetine for weight reduction in obese women: a preliminary randomised controlled trial. Int J Obes 30(7):1138–1142
McElroy S, Guerdjikova A, Kotwal R, Welge J, Nelson E, Lake K et al (2007) Atomoxetine in the treatment of binge-eating disorder: a randomized placebo-controlled trial. J Clin Psychiatry 68(3):390–398
Pott W, Albayrak O, Hinney A, Hebebrand J, Pauli-Pott U (2013) Successful treatment with atomoxetine of an adolescent boy with attention deficit/hyperactivity disorder, extreme obesity, and reduced melanocortin 4 receptor function. Obes Facts 6(1):109–115
Samardzic J, Allegaert K, Bajcetic M (2015) Developmental pharmacology: a moving target. Int J Pharm 492(1-2):335–337
Kearns G, Abdel-Rahman S, Alander S, Blowey D, Leeder J, Kauffman R (2003) Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Hines R, McCarver D (2002) The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther 300(2):355–360
Stevens J, Marsh S, Zaya M, Regina K, Divakaran K, Le M et al (2008) Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos 36(8):1587–1593
Blake M, Gaedigk A, Pearce R, Bomgaars L, Christensen M, Stowe C et al (2007) Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther 81(4):510–516
Strolin Benedetti M, Whomsley R, Baltes E (2005) Differences in absorption, distribution, metabolism and excretion of xenobiotics between the paediatric and adult populations. Expert Opin Drug Metab Toxicol 1(3):447–471
Verscheijden L, Koenderink J, Johnson T, de Wildt S, Russel F (2020) Physiologically-based pharmacokinetic models for children: starting to reach maturation? Pharmacol Ther 211:107541
Upadhyaya H, Kratochvil C, Ghuman J, Camporeale A, Lipsius S, D’Souza D et al (2015) Efficacy and safety extrapolation analyses for atomoxetine in young children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 25(10):799–809
van Groen B, Nicolaï J, Kuik A, Van Cruchten S, van Peer E, Smits A et al (2021) Ontogeny of hepatic transporters and drug-metabolizing enzymes in humans and in nonclinical species. Pharmacol Rev 73(2):597–678
Bebia Z, Buch S, Wilson J, Frye R, Romkes M, Cecchetti A et al (2004) Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther 76(6):618–627
Kinirons M, Crome P (1997) Clinical pharmacokinetic considerations in the elderly. An update. Clin Pharmacokinet 33(4):302–312
Pritchard J, Bryson J, Kernodle A, Benedetti T, Powell J (1992) Age and gender effects on ondansetron pharmacokinetics: evaluation of healthy aged volunteers. Clin Pharmacol Ther 51(1):51–55
Gex-Fabry M, Balant-Gorgia A, Balant L, Garrone G (1990) Clomipramine metabolism. Model-based analysis of variability factors from drug monitoring data. Clin Pharmacokinet 19(3):241–255
Hägg S, Spigset O, Dahlqvist R (2001) Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol 51(2):169–173
Hooper W, Qing M (1990) The influence of age and gender on the stereoselective metabolism and pharmacokinetics of mephobarbital in humans. Clin Pharmacol Ther 48(6):633–640
Richardson C, Blocka K, Ross S, Verbeeck R (1985) Effects of age and sex on piroxicam disposition. Clin Pharmacol Ther 37(1):13–18
Kratochvil C, Milton D, Vaughan B, Greenhill L (2008) Acute atomoxetine treatment of younger and older children with ADHD: a meta-analysis of tolerability and efficacy. Child Adolesc Psychiatry Ment Health 2(1):25
Fu D, Wu D, Guo H, Hu Y, Xia Y, Ji X et al (2021) The mechanism, clinical efficacy, safety, and dosage regimen of atomoxetine for ADHD therapy in children: a narrative review. Front Psych 12:780921
Wilens T, Kratochvil C, Newcorn J, Gao H (2006) Do children and adolescents with ADHD respond differently to atomoxetine? J Am Acad Child Adolesc Psychiatry 45(2):149–157
Belle DJ, Ernest CS, Sauer JM, Smith BP, Thomasson HR, Witcher JW (2002) Effect of potent CYP2D6 inhibition by paroxetine on atomoxetine pharmacokinetics. J Clin Pharmacol 42(11):1219–1227
Pliszka SR (2007) Pharmacologic treatment of attention-deficit/hyperactivity disorder: efficacy, safety and mechanisms of action. Neuropsychol Rev 17(1):61–72
Pohl GM, Van Brunt DL, Ye W, Stoops WW, Johnston JA (2009) A retrospective claims analysis of combination therapy in the treatment of adult attention-deficit/hyperactivity disorder (ADHD). BMC Health Serv Res 9:95
Todor I, Popa A, Neag M, Muntean D, Bocsan C, Buzoianu A et al (2016) Evaluation of a potential metabolism-mediated drug-drug interaction between atomoxetine and bupropion in healthy volunteers. J Pharm Pharm Sci 19(2):198–207
Hazell P, Becker K, Nikkanen E, Trzepacz P, Tanaka Y, Tabas L et al (2009) Relationship between atomoxetine plasma concentration, treatment response and tolerability in attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Atten Defic Hyperact Disord 1(2):201–210
Jing Y, Kong Y, Hou X, Liu H, Fu Q, Jiao Z et al (2021) Population pharmacokinetic analysis and dosing guidelines for tacrolimus co-administration with Wuzhi capsule in Chinese renal transplant recipients. J Clin Pharm Ther 46(4):1117–1128
Acknowledgements
This research was supported by the Specially Appointed Medical Expert Project of the Jiangsu Commission of Health (2019), the Talent Project established by the Chinese Pharmaceutical Association Hospital Pharmacy department (NO. CPA-Z05-ZC-2022-003), a grant from Jiangsu Research Hospital Association for Precision Medication (JY2022).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.X., D.D.W., and F.C.; writing—original draft preparation, D.F.; writing—review and editing, D.F., F.C., H.-L.G., Y.-H.H., W.-R.F., and Q.-Q.L.; critical revision of the manuscript, F.C; funding acquisition, F.C. All authors have read and agreed to the submitted version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Di Fu is a visiting graduate student from China Pharmaceutical University.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, D., Guo, HL., Hu, YH. et al. Personalizing atomoxetine dosing in children with ADHD: what can we learn from current supporting evidence. Eur J Clin Pharmacol 79, 349–370 (2023). https://doi.org/10.1007/s00228-022-03449-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03449-1