Abstract
Purpose
The aim of this study was to assess polymyxin B pharmacokinetics (PK) in patients with varying degrees of renal dysfunction and in patients who require continuous veno-venous hemodialysis (CVVHD).
Methods
The study enrolled 37 patients with sepsis, including 13 patients with glomerular filtration rate (GFR) below 80 mL/min and 11 patients on CVVHD. Each patient received a loading dose of polymyxin B (200–300 mg) and at least 3 subsequent doses of 100–150 mg every 12 h. For every patient, 6–8 blood samples were collected between doses. Polymyxin B (PMB) serum concentration was determined using enzyme-linked immunosorbent assay.
Results
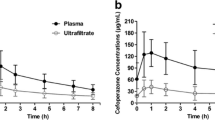
In sepsis, patients with preserved renal function mean area under the curve over 24 h (AUC0–24 h) value reached 67.8 ± 9.8 mg*h/L, while in patients with GFR below 80 mL/min, mean AUC0–24 h was 87 ± 5.8 mg*h/L. PMB PK in patients with renal insufficiency was characterized by significantly lower clearance (CL) compared to the normal renal function group (2.1 ± 0.1 L/h vs 3.9 ± 0.4 L/h respectively). In patients on CVVHD, mean AUC0–24 h was 110.4 ± 10.3 mg*h/L, while CL reached 2 ± 0.23 L/h. The median recovery rate from dialysate constituted 22%. Simulation of different dosage regimens that indicate a fixed maintenance dose of 100 mg q12h with a loading dose of 200 mg is optimal for patients on CVVHD, and no dosage increase is required.
Conclusion
This study demonstrates decreased clearance of PMB in patients with renal insufficiency, which puts them at risk of toxicity. Therefore, patients with extremes of renal function might benefit from therapeutic drug monitoring. For patients with anuria, who require CVVHD, we suggest a fixed dose of 100 mg q12h.


Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Falagas ME, Lourida P, Poulikakos P et al (2014) Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58(2):654–663
Velkov T, Roberts KD, Nation RL et al (2013) Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol 8(6):711–724
Tsuji BT, Pogue JM, Zavascki AP et al (2019) International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39(1):10–39
Avedissian SN, Liu J, Rhodes NJ et al (2019) A review of the clinical pharmacokinetics of polymyxin B. Antibiotics (Basel) 8(1):31
Bergen PJ, Li J, Rayner CR, Nation RL (2006) Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50(6):1953–1958
Manchandani P, Zhou J, Ledesma KR et al (2016) Characterization of polymyxin B biodistribution and disposition in an animal model. Antimicrob Agents Chemother 60(2):1029–1034
Nang SC, Azad MAK, Velkov T et al (2021) Rescuing the last-line polymyxins: achievements and challenges. Pharmacol Rev 73(2):679–728
Bagshaw SM, George C, Bellomo R (2008) Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 12(2):R47
Bagshaw SM, Uchino S, Bellomo R et al (2007) Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2(3):431–439
Pogue JM, Tam VH (2019) Toxicity in Patients. In: Li J, Nation R, Kaye K (eds) Polymyxin antibiotics: from laboratory bench to bedside. Adv Exp Med Biol vol 1145. Springer, Cham. https://doi.org/10.1007/978-3-030-16373-0_17
Lakota EA, Landersdorfer CB, Nation RL et al (2018) Personalizing polymyxin B dosing using an adaptive feedback control algorithm. Antimicrob Agents Chemother 62(7):e00483–e518
Sandri AM, Landersdorfer CB, Jacob J et al (2013) Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57(4):524–531
Kubin CJ, Nelson BC, Miglis C et al (2018) Population pharmacokinetics of intravenous polymyxin B from clinical samples. Antimicrob Agents Chemother 62(3):e01493–e1517
Thamlikitkul V, Dubrovskaya Y, Manchandani P et al (2017) Dosing and pharmacokinetics of polymyxin B in Patients with renal insufficiency. Antimicrob Agents Chemother 61(1):e01337–e1416
Wang P, Zhang Q, Zhu Z et al (2021) Comparing the population pharmacokinetics of and acute kidney injury due to polymyxin B in Chinese patients with or without renal insufficiency. Antimicrob Agents Chemother 65(2):e01900–e1920
Honoré PM, Jacobs R, Joannes-Boyau O et al (2014) Continuous renal replacement therapy-related strategies to avoid colistin toxicity: a clinically orientated review. Blood Purif 37(4):291–295
Nation RL, Garonzik SM, Thamlikitkul V et al (2017) Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 64(5):565–571
Choi G, Gomersall CD, Tian Q et al (2009) Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med 37(7):2268–2282
Luo X, Zhang Y, Liang P et al (2022) Population pharmacokinetics of polymyxin B and dosage strategy in critically ill patients with/without continuous renal replacement therapy. Eur J Pharm Sci 175:106214
Wang P, Xing H, Zhang F et al (2022) Population pharmacokinetics of polymyxin B in critically ill patients receiving continuous venovenous haemofiltration. Int J Antimicrob Agents 60(1):106599
Sandri AM, Landersdorfer CB, Jacob J et al (2013) Pharmacokinetics of polymyxin B in patients on continuous venovenous haemodialysis. J Antimicrob Chemother 68(3):674–677
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Burkin MA, Galvidis IA, Surovoy YA et al (2021) Development of ELISA formats for polymyxin B monitoring in serum of critically ill patients. J Pharm Biomed Anal 204:114275
Rigatto MH, Falci DR, Lopes NT et al (2016) Clinical features and mortality of patients on renal replacement therapy receiving polymyxin B. Int J Antimicrob Agents 47(2):146–150
Acknowledgements
The authors acknowledge the personnel of Clinical Hospital #1 MEDSI intensive care unit for their help with sample and data collection.
Author information
Authors and Affiliations
Contributions
YAS: writing—original draft preparation, PK, and statistical analysis. MAB: conceptualization, formal analysis and investigation, review, and editing. IAG: methodology, formal analysis, and validation. MAS: sample collection, PK, and statistical analysis. OCR: sample collection, PK, and statistical analysis. SVT: supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. This study was approved by MEDSI Clinic Independent Ethical Committee, Moscow, Russia (Protocol #29 April 15, 2021).
Consent to participate
Informed consent form was signed by legal representatives of the patients.
Consent for publication
Informed consent form was signed by legal representatives of the patients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Keypoints
• Acute kidney injury has significant impact on polymyxin B clearance, and therapeutic drug monitoring might be beneficial in certain groups of critically ill patients.
• Continuous veno-venous hemodialysis does not lead to significant removal of polymyxin B; therefore, a fixed dosage regimen of 100 mg twice daily might be appropriate in these patients.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Surovoy, Y.A., Burkin, M.A., Galvidis, I.A. et al. Comparative polymyxin B pharmacokinetics in critically ill patients with renal insufficiency and in continuous veno-venous hemodialysis. Eur J Clin Pharmacol 79, 79–87 (2023). https://doi.org/10.1007/s00228-022-03415-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03415-x