Abstract
Purpose
Anemia of chronic kidney disease (CKD) has traditionally been treated with recombinant human erythropoietin (rhEPO). Recently, daprodustat, a hypoxia-inducible factor prolyl-hydroxylase inhibitor, has also been shown to increase hematocrit. It remains unclear whether daprodustat or rhEPO should be the treatment of choice for anemia of CKD. We aimed to assess the efficacy and cardiovascular safety of daprodustat versus rhEPO in CKD patients.
Methods
Online databases were queried in April 2022 for articles comparing the efficacy and safety of daprodustat in DD-CKD and NDD-CKD subgroups. Results from trials were pooled using a random-effects model.
Results
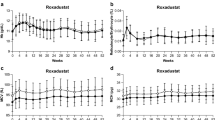
Data on 8245 CKD patients from eight clinical trials were included. Our results show that in comparison to rhEPO, daprodustat maintained the same efficacy in increasing hemoglobin levels in both the DD-CKD (MD: 0.10; 95% CI [− 0.13,0.34]; p = 0.50) and NDD-CKD (MD: − 0.01; 95% CI [− 0.38,0.35]; p = 0.95) subgroups. Daprodustat significantly lowered hepcidin levels and significantly increased TIBC in both subgroups. Additionally, daprodustat significantly reduced the incidence of major adverse cardiovascular events (MACE) (RR: 0.89; 95% CI: 0.89–0.98; p = 0.02) and its myocardial infarction (MI) component (RR: 0.74; 95% CI: 0.59–0.92; p = 0.006) in the DD-CKD subgroup.
Conclusion
Daprodustat has similar efficacy compared to rhEPO for the treatment of anemia of CKD. On treatment, the reduced experience of MACE was reported in DD-CKD patients as compared to rhEPO. Furthermore, effects on iron metabolism varied by parameter, with daprodustat being superior to rhEPO in some cases and inferior in others.


Similar content being viewed by others
Availability of data materials
The data that support the findings of this study were sourced directly from the published studies included in this systematic review and meta-analysis.
References
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, Adebayo OM, Afarideh M, Agarwal SK, Agudelo-Botero M, Ahmadian E (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 395(10225):709–33
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M et al (2021) 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With. Eur Heart J 42(34):3227–3337
Portolés J, Martín L, Broseta JJ, Cases A (2021) Anemia in chronic kidney disease: from pathophysiology and current treatments, to Future Agents. Front Med 26(8):328
Shepshelovich D, Rozen-Zvi B, Avni T, Gafter U, Gafter-Gvili A (2016) Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis 68(5):677–690
McMurray J, Parfrey P, Adamson JW, Aljama P, Berns JS, Bohlius J et al (2012) Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 279–335
US Food and Drug Administration (2011) FDA drug safety communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease
Bonomini M, Del Vecchio L, Sirolli V, Locatelli F (2016) New treatment approaches for the anemia of CKD. Am J Kidney Dis 67(1):133–142
Locatelli F, Bárány P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D et al (2013) Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant 28(6):1346–1359
Zheng Q, Wang Y, Yang H, Sun L, Fu X, Wei R et al (2020) Efficacy and safety of daprodustat for anemia therapy in chronic kidney disease patients: a systematic review and meta-analysis. Front Pharmacol 11:573645
Patoulias D, Papadopoulos C, Doumas M (2022) Meta-analysis addressing the cardiovascular safety of daprodustat in patients with chronic kidney disease undergoing dialysis or not. Am J Cardiol 170:166–167
Singh AK, Cizman B, Carroll K, McMurray JJ V, Perkovic V, Jha V et al (2022) Efficacy and safety of daprodustat for treatment of anemia of chronic kidney disease in incident dialysis patients: a randomized clinical trial. JAMA Intern Med
amstar-2_-a-critical-appraisal-tool-for-systematic-reviews-that-include-randomised-or-non-randomised-studies-of-healthcare-interventions,-or-both. Available from: https://www.bmj.com/content/358/bmj.j4008
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) (2022) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. Available from www.training.cochrane.org/handbook
Rohatgi A (2021) WebPlotDigitizer Version 4.5 [Internet]. Available from: https://automeris.io/WebPlotDigitizer
Burda BU, O’Connor EA, Webber EM, Redmond N, Perdue LA (2017) Estimating data from figures with a web-based program: considerations for a systematic review. Res Synth Methods [Internet] 8(3):258–62. Available from: https://pubmed.ncbi.nlm.nih.gov/28268241/. [cited 2022 Mar 8]
Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC (2019) Assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews of Interventions p. 205–28. Wiley Online Books
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Singh AK, Carroll K, Perkovic V, Solomon S, Jha V, Johansen KL et al (2021) Daprodustat for the treatment of anemia in patients undergoing dialysis. N Engl J Med 385(25):2325–2335
Singh AK, Carroll K, McMurray JJV, Solomon S, Jha V, Johansen KL et al (2021) Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med 385(25):2313–2324
Holdstock L, Meadowcroft AM, Maier R, Johnson BM, Jones D, Rastogi A et al (2016) Four-week studies of oral hypoxia-inducible factor–prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol [Internet] 27(4):1234–44. Available from: https://pubmed.ncbi.nlm.nih.gov/26494831/. [cited 2022 Apr 27]
Meadowcroft AM, Cizman B, Holdstock L, Biswas N, Johnson BM, Jones D et al (2019) Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J 12(1):139–148
Akizawa T, Nangaku M, Yonekawa T, Okuda N, Kawamatsu S, Onoue T et al (2020) Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol 15(8):1155–1165
Nangaku M, Hamano T, Akizawa T, Tsubakihara Y, Nagai R, Okuda N et al (2021) Daprodustat compared with epoetin beta pegol for anemia in Japanese patients not on dialysis: a 52-week randomized open-label phase 3 trial. Am J Nephrol 52(1):26–35
Holdstock L, Cizman B, Meadowcroft AM, Biswas N, Johnson BM, Jones D et al (2019) Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J 12(1):129–138
Fu Z, Geng X, Chi K, Song C, Wu D, Liu C et al (2022) Efficacy and safety of daprodustat vs rhEPO for anemia in patients with chronic kidney disease: a meta-analysis and trial sequential analysis. Front Pharmacol 13
Coyne DW (2011) Hepcidin: clinical utility as a diagnostic tool and therapeutic target. Kidney Int [Internet] 80(3):240–4. Available from: http://www.kidney-international.org/article/S0085253815550345/fulltext. [cited 2022 Apr 27]
Ikeda-Taniguchi M, Takahashi K, Shishido K, Honda H (2022) Total iron binding capacity is a predictor for muscle loss in maintenance hemodialysis patients. Clin Exp Nephrol [Internet] 26(6):583–92. https://doi.org/10.1007/s10157-022-02193-1. [cited 2022 Sep 20]
Stack AG, Mutwali AI, Nguyen HT, Cronin CJ, Casserly LF, Ferguson J (2014) Transferrin saturation ratio and risk of total and cardiovascular mortality in the general population. QJM An Int J Med [Internet] 107(8):623–33. Available from: https://academic.oup.com/qjmed/article/107/8/623/2948338. [cited 2022 Sep 20]
Kuragano T, Joki N, Hase H, Kitamura K, Murata T, Fujimoto S et al (2020) Low transferrin saturation (TSAT) and high ferritin levels are significant predictors for cerebrovascular and cardiovascular disease and death in maintenance hemodialysis patients. PLoS One [Internet] 15(9). Available from: https://pubmed.ncbi.nlm.nih.gov/32877424/. [cited 2022 Apr 30]
Author information
Authors and Affiliations
Contributions
K.F conceptualized and, together with T.A, supervised the investigation. Search strings were designed by A.S.F, and abstract and title screening, as well as full-text review screening, were performed by W.A and M.U.M with conflicts being resolved by A.S.F. Data extraction was performed independently by M.B.I and M.T.N, with conflicts being resolved by A.A. W.A and M.U.M were major contributors in analyzing the data. Quality assessment and certainty of evidence assessment were performed independently by W.A and M.T.M, with conflicts being resolved by A.S.F. Supplementary material was prepared by A.S.F, M.B.I, M.T.M, M.T.N, A.A, and S.R.A. W.A, O.M, A.S.F, and S.R.A were major contributors in writing and editing the manuscript. All authors critically reviewed the results, approved the final manuscript for publication, and agree to be accountable for all aspects of the work done in producing this manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was exempt from full ethical approval by an institutional review board as no original data was included. There were no interactions with any human or animal participants across the duration of this study.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fatima, K., Ahmed, W., Fatimi, A.S. et al. Evaluating the safety and efficacy of daprodustat for anemia of chronic kidney disease: a meta-analysis of randomized clinical trials. Eur J Clin Pharmacol 78, 1867–1875 (2022). https://doi.org/10.1007/s00228-022-03395-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03395-y