Abstract
Purpose
To assess the feasibility and acceptance of the semi-automated meta-analysis (SAMA). The objectives are twofold, namely (1) to compare expert opinion on the quality of protocols, methods, and results of one conventional meta-analysis (CMA) and one SAMA and (2) to compare the time to execute the CMA and the SAMA.
Methods
Experts evaluated the protocols and manuscripts/reports of the CMA and SAMA conducted independently on the safety of metronidazole in pregnancy. Expert opinion was collected using AMSTAR 2 checklist. Time spent was recorded using case report forms.
Results
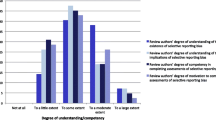
The overall scores of the opinion of all experts for protocols, methods, and results for SAMA (6.75) and CMA (6.87) were not statistically different (p = 0.88). The experts’ confidence in the results of each MA was 7.89 ± 1.17 and 8.11 ± 0.92, respectively. The time to completion was 14 working days for SAMA and 24.7 for CMA. MA tasks such as calculation of effect estimates, subgroup/sensitivity analysis, and publication bias investigation required no investment in time for SAMA.
Conclusion
In conclusion, our study demonstrated the feasibility of SAMA and suggests acceptance for risk assessment by an expert committee. Our results suggest that SAMA reduces the time required for a MA without altering expert confidence in the methodological and scientific rigor. As our study was limited to one example, the generalization of our results requires confirmation by other studies.


Similar content being viewed by others
References
Haidich AB (2010) Meta-analysis in medical research. Hippokratia 14(Suppl 1):29–37
Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G (2013) Meta-analysis: pitfalls and hints. Heart Lung Vessels 5(4):219–225
Bastian H, Glasziou P, Chalmers I (2010) Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med 7(9):e1000326
Allen IE, Olkin I (1999) Estimating time to conduct a meta-analysis from number of citations retrieved. JAMA 282(7):634–635
Borah R, Brown AW, Capers PL, Kaiser KA (2017) Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open 7(2):e012545
Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S et al (2017) Living systematic review: 1. Introduction—the why, what, when, and how. J Clin Epidemiol 91:23–30
Thomas J, Noel-Storr A, Marshall I, Wallace B, McDonald S, Mavergames C et al (2017) Living systematic reviews: 2. Combining human and machine effort. J Clin Epidemiol 91:31–37
Safi S, Thiessen T, Schmailzl KJ (2018) Acceptance and resistance of new digital technologies in medicine: qualitative study. JMIR Res Protoc 7(12):e11072
O’Connor AM, Tsafnat G, Thomas J, Glasziou P, Gilbert SB, Hutton B (2019) A question of trust: can we build an evidence base to gain trust in systematic review automation technologies? Syst Rev 8:143
Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM (2020) A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol 121:81–90
O’Connor AM, Tsafnat G, Gilbert SB, Thayer KA, Wolfe MS (2018) Moving toward the automation of the systematic review process: a summary of discussions at the second meeting of International Collaboration for the Automation of Systematic Reviews (ICASR). Syst Rev 7(1):3
Marshall IJ, Wallace BC (2019) Toward systematic review automation: a practical guide to using machine learning tools in research synthesis. Syst Rev 8(1):163
Gates A, Gates M, DaRosa D, Elliott SA, Pillay J, Rahman S et al (2020) Decoding semi-automated title-abstract screening: findings from a convenience sample of reviews. Syst Rev 9(1):272
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [Accessed 13 févr 2020];358. https://www.bmj.com/content/358/bmj.j4008
Pussegoda K, Turner L, Garritty C, Mayhew A, Skidmore B, Stevens A et al (2017) Identifying approaches for assessing methodological and reporting quality of systematic reviews: a descriptive study. Syst Rev 6(1):117
Hemming V, Burgman MA, Hanea AM, McBride MF, Wintle BC (2018) A practical guide to structured expert elicitation using the IDEA protocol. Methods Ecol Evol 9(1):169–180
Speirs-Bridge A, Fidler F, McBride M, Flander L, Cumming G, Burgman M (2010) Reducing overconfidence in the interval judgments of experts. Risk Anal Off Publ Soc Risk Anal 30(3):512–523
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Internet]. 2020 [Accessed 30 mars 2020] https://www.r-project.org/
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36(1):1–48
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22(4):153–160
Collignon A, Cuxac P (2017) ISTEX: des enrichissements au web de données. I2D - Inf Donnees Doc 54(4):8‑15
Higgins JP, Green S (2008) Cochrane Handbook for Systematic Reviews of Interventions: Chichester, UK: John Wiley & Sons, Ltd. http://doi.wiley.com/10.1002/9780470712184
van Altena AJ, Spijker R, Olabarriaga SD (2019) Usage of automation tools in systematic reviews. Res Synth Methods 10(1):72–82
Chai KEK, Lines RLJ, Gucciardi DF et al (2021) Research screener: a machine learning tool to semi-automate abstract screening for systematic reviews | SpringerLink [Internet]. [Accessed 28 avr 2021]. https://link.springer.com/article/10.1186/s13643-021-01635-3
Low J, Ross JS, Ritchie JD, Gross CP, Lehman R, Lin H et al (2017) Comparison of two independent systematic reviews of trials of recombinant human bone morphogenetic protein-2 (rhBMP-2): the Yale Open Data Access Medtronic Project. Syst Rev 6(1):28
Wiseman MJ (2008) Reproducibility of systematic literature reviews on food, nutrition, physical activity and endometrial cancer. Public Health Nutrition, 11 (10). 1006 - 1014 . ISSN 1368-9800 https://doi.org/10.1017/S1368980007001334 [Accessed 24 févr 2021] https://core.ac.uk/reader/13505414?utm_source=linkout
Acknowledgements
We thank the following experts of the ANSM Scientific Expert Committee for their contribution to this project:
Dr Florence Gressier MD PhD HDR: CESP, Inserm UMR1178, Department of Psychiatry, Assistance Publique-Hôpitaux de Paris, Bicêtre University Hospital, Le Kremlin Bicêtre, France. Dr Isabelle Lacroix: REGARDS Network, Medical and Clinical Pharmacology Department, Midi-Pyrenees Regional Centre for Pharmacovigilance, Pharmacoepidemiology and Drug Information (CRPV), Toulouse University Hospital, University of Toulouse, Inserm 1027. Dr Kim an Nguyen: Department of Pharmacotoxicology, Clinical Investigation Centre 1407, Inserm, Hospices Civils de Lyon, 69003 Lyon, France; UMR5558 CNRS, Laboratoire de Biométrie et Biologie Evolutive, Université de Lyon, 69008 Lyon, France. Dr Nadine Saleh PharmD MS PhD: Head, Master of Public Health, Faculty of Public Health, Lebanese University, Lebanon. Dr Sophie Gautier: Centre Regional de Pharmacovigilance, Centre Hospitalier Universitaire de Lille, Lille, France. Dr Thierry Vial MD: Service Hospitalo-Universitaire de Pharmacotoxicologie, CHU-Lyon, Lyon, France.
Author information
Authors and Affiliations
Contributions
PA: conceptualization, methodology, validation, visualization, formal analysis, data curation, writing — original draft preparation. JC: conceptualization, data curation, validation, writing — reviewing and editing. CP: data curation, validation, reviewing and editing. AU: data curation, validation. ER: conceptualization, validation. MC: conceptualization, software. PM: conceptualization, methodology, validation, writing — reviewing and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ajiji, P., Cottin, J., Picot, C. et al. Feasibility study and evaluation of expert opinion on the semi-automated meta-analysis and the conventional meta-analysis. Eur J Clin Pharmacol 78, 1177–1184 (2022). https://doi.org/10.1007/s00228-022-03329-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03329-8