Abstract
Objective
This study evaluated the pharmacokinetic (PK) characteristics of benapenem in subjects with mild to moderate renal impairment to provide a reference for benapenem dosing regimens in this patient population.
Methods
Eighteen subjects were enrolled in this study. Each subject received a single dose of benapenem intravenously (1.0 g in 100 ml of 0.9% saline) followed by blood and urine collection to measure the concentrations of benapenem and its major metabolite. PK analysis was performed to evaluate the effect of varying degrees of renal impairment on the PK characteristics of benapenem. The safety of benapenem was also evaluated.
Results
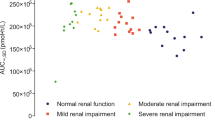
In subjects with normal renal function, mild renal impairment, and moderate renal impairment, the maximum plasma benapenem concentrations were 163 ± 6.58 mg/L, 138 ± 17.4 mg/L, and 134 ± 0.11 mg/L, respectively (15.3% and 17.8% lower in subjects with mild and moderate renal impairment, respectively, than in subjects with normal renal function). The areas under the plasma concentration–time curve (AUC0-inf) were 1153.67 ± 143.2 mg·h/L, 1129.17 ± 241.41 mg·h/L, and 1316.46 ± 229.83 mg·h/L, respectively (P > 0.05); the cumulative urinary excretion rates at 72 h after dosing were 52.61 ± 8.58%, 39.42 ± 8.35%, and 29.84 ± 9.15%, respectively; and the metabolic ratio (AUC0-inf_KBP-3331/AUC0-inf_benapenem) were 3.96 ± 0.35%, 5.56 ± 0.82%, and 8.24 ± 0.85%, respectively. No drug-related adverse events (AEs), serious AEs, or AEs leading to withdrawal occurred in this study.
Conclusion
No adjustment to benapenem dosing is needed in patients with mild to moderate renal impairment.
Clinical trial registration
Drug clinical trial registration and information publicity platform: http://www.chinadrugtrials.org.cn/index.html. Registration number: CTR20190760.


Similar content being viewed by others
Data availability
The data in this article belong to the sponsor. The data may be made available to readers with permission from the corresponding author and sponsor.
References
Baughman RP (2009) The use of carbapenems in the treatment of serious infections. J Intensive Care Med 24:230–241. https://doi.org/10.1177/0885066609335660
Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA (2016) Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 176:1639–1648. https://doi.org/10.1001/jamainternmed.2016.5651
Lu X, Li CR, Pang J, Hu XX, Nie TY, Yang XY et al (2015) Pharmacodynamic evaluation of benapenem, a new carbapenem antibiotic, in mouse urinary tract infection model. Chin Med Biotechnol 10:139–143. https://doi.org/10.3969/cmba.j.issn.1673-713X.2015.02.008
Zhao CY, Lv Y, Zhu Y, Wei MJ, Liu MY, Ji XW et al (2019) A first-in-human safety, tolerability, and pharmacokinetics study of benapenem in healthy Chinese volunteers. Antimicrob Agents Chemother 63:e02188-e2218. https://doi.org/10.1128/aac.02188-18
Afshartous D, Bauer SR, Connor MJ, Aduroja OA, Amde M, Salem C et al (2014) Pharmacokinetics and pharmacodynamics of imipenem and meropenem in critically ill patients treated with continuous venovenous hemodialysis. Am J Kidney Dis 63:170–171. https://doi.org/10.1053/j.ajkd.2013.08.015
Suzuki H, Tokuda Y, Shichi D, Hitomi S, Ishikawa H, Maeno T et al (2013) A retrospective cohort study of panipenem/betamipron for adult pneumococcal bacteremia at three teaching hospitals in Japan. J Infect Chemother 19:607–614. https://doi.org/10.1007/s10156-012-0525-1
Hang Y, Chen Y, Xue L, Sun S, Liu L, Gao J et al (2018) Evaluating biapenem dosage regimens in intensive care unit patients with Pseudomonas aeruginosa infections: a pharmacokinetic/pharmacodynamic analysis using Monte Carlo simulation. Int J Antimicrob Agents 51:484–487. https://doi.org/10.1016/j.ijantimicag.2017.07.005
Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG et al (2016) Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 63:754–762. https://doi.org/10.1093/cid/ciw378
El-Gamal MI, Brahim I, Hisham N, Aladdin R, Mohammed H, Bahaaeldin A (2017) Recent updates of carbapenem antibiotics. Eur J Med Chem 131:185–195. https://doi.org/10.1016/j.ejmech.2017.03.022
Ji XW, Xue F, Kang ZS, Zhong W, Kuan IH, Yang XP et al (2020) Model-informed drug development, pharmacokinetic/pharmacodynamic cutoff value determination, and antibacterial efficacy of benapenem against enterobacteriaceae. Antimicrob Agents Chemother 64:e01751-e1819. https://doi.org/10.1128/aac.01751-19
Chinese eGFR Investigation Collaboration (2006) Modification and evaluation of MDRD estimating equation for Chinese patients with chronic kidney disease. Chin J Nephrol 22:589–595
Ji X, Kang Z, Li Y, Yang X, Ma X, Shi C et al (2019) An established LC-MS/MS method and a developed PK model for the study of pharmacokinetic properties of benapenem in infected mice. J Chin Pharm Sci 28:802–811. https://doi.org/10.5246/jcps.2019.11.076
U. S. Department of Health and Human Services (2017) Common terminology criteria for adverse events (CTCAE) version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc. htm#ctc_50
Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S et al (2013) Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans. Antimicrob Agents Chemother 57:3536–3546. https://doi.org/10.1128/aac.00292-13
National Medical Products Administration (2012) Technical guidelines for pharmacokinetic studies in patients with impaired renal function. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20120515120001975.html
Merck Sharp & Dohme Limited (2019) Instructions for ertapenem for injection. https://www.msdchina.com.cn/product/chufangyao.html
Neo HY, Tan KT, Caroline C, Ng DWH, Ho EPY, Lim JP et al (2020) Higher rates of carbapenem-related seizures in older hospitalised adults. Intern Med J 50:123–127. https://doi.org/10.1111/imj.14693
Miller AD, Ball AM, Bookstaver PB, Dornblaser EK, Bennett CL (2011) Epileptogenic potential of carbapenem agents: mechanism of action, seizure rates, and clinical considerations. Pharmacotherapy 31:408–423. https://doi.org/10.1592/phco.31.4.408
Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA (2014) The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother 69:2043–2055. https://doi.org/10.1093/jac/dku111
Roffey SJ, Obach RS, Gedge JI, Smith DA (2007) What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metab Rev 39:17–43. https://doi.org/10.1080/03602530600952172
Acknowledgements
We sincerely thank the researchers at the Phase I Unit and Department of Nephrology, Huashan Hospital, Fudan University, the Phase I Unit and Department of Nephrology, Shanghai Tongji Hospital, and the Department of Nephrology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine for their contributions to this study.
Funding
This work was supported by a grant from Shanghai municipal Human Resources and Social Security Bureau (no. 03) and the New Drug Creation and Manufacturing Program of the Ministry of Science and Technology of China (no. 2017ZX09304005). The clinical trial was sponsored by Xuanzhu Co., Ltd.
Author information
Authors and Affiliations
Contributions
HY and MZ coordinated the clinical trial and wrote the manuscript. JZ, JX, HR, HZ, and CY designed and performed the research. CX and ZH participated in the design of the research. YC participated in the analysis and interpretation of the data. JL, LY, JY, HL and JW performed the clinical trial.
Corresponding authors
Ethics declarations
Conflict of interest
CX is a full-time employee of Xuanzhu Biopharmaceutical Co., Ltd. ZH was the employee of Xuanzhu Co., Ltd when the study was ongoing. Other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, H., Zhang, M., Chen, Y. et al. Pharmacokinetics of benapenem for injection in subjects with mild to moderate renal impairment. Eur J Clin Pharmacol 78, 1079–1086 (2022). https://doi.org/10.1007/s00228-022-03317-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03317-y