Abstract
Purpose
Anti-neutrophilic cytoplasmic antibody (ANCA)–associated vasculitis is a rare autoimmune disease. Mycophenolic acid (MPA) is widely used for ANCA-associated nephritis (AAN) but with large pharmacokinetic variability. This study aims to investigate clinical factors impacting MPA disposition in pediatric AAN.
Methods
We retrospectively collected 391 MPA concentrations from 25 children diagnosed with AAN. A population pharmacokinetic model was developed to explore the potential effects of demographics and biochemical covariates on MPA. Monte Carlo simulations were performed to optimize dosage regimen.
Results
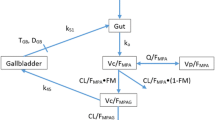
MPA pharmacokinetics best fitted a two-compartment model with first-order absorption and linear elimination. The pharmacokinetic parameters for Ka, CL/F, Vc/F, Vp/F, and Q/F were 0.45 h−1, 9.86 L/h, 19.69 L, 408.32 L, and 23.01 L/h. Dosage form significantly affected drug absorption. CL/F significantly decreased with increasing cystatin C, while decreasing with myeloperoxidase. Cystatin C was superior to serum creatinine in predicting apparent clearance of MPA. A dose regimen of 650 mg/m2 twice daily was required to achieve target exposure in children with normal renal function and no inflammation. The combined effects of myeloperoxidase concentration and renal function resulted in a sixfold range of MPA dose.
Conclusion
This was the first study of MPA population pharmacokinetic model in children with AAN. Myeloperoxidase was not only a biomarker of AAN, but also an inflammatory factor to impact drug CL. The influence of renal function and underlying diseases on drug metabolism should be fully considered in personalized medication for AAN.




Similar content being viewed by others
Availability of data and material
The data used to support the findings of this study are available from the corresponding author on reasonable request.
References
Geetha D, Jefferson JA (2020) ANCA-associated vasculitis: core curriculum. Am J Kidney Dis 75(1):124–137
Haris Á, Dolgos S, Polner K (2017) Therapy and prognosis of ANCA-associated vasculitis from the clinical nephrologist’s perspective. Int Urol Nephrol 49(1):91–102
Yates M, Watts RA, Bajema IM et al (2016) EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 75(9):1583–1594
Jones RB, Hiemstra TF, Ballarin J et al (2019) Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis 78(3):399–405
Kiang TKL, Ensom MHH (2018) Population pharmacokinetics of mycophenolic acid: an update. Clin Pharmacokinet 57(5):547–558
Joy MS, Hilliard T, Hu Y, Hogan SL, Wang J, Falk RJ, Smith PC (2009) Influence of clinical and demographic variables on mycophenolic acid pharmacokinetics in antineutrophil cytoplasmic antibody-associated vasculitis. Ann Pharmacother 43(6):1020–1027
Schaier M, Scholl C, Scharpf D, Schmitt WH, Schwenger V, Zeier M, Sommerer C (2015) High interpatient variability in response to mycophenolic acid maintenance therapy in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 30(Suppl 1):i138-145
Chaigne B, Gatault P, Darrouzain F et al (2014) Mycophenolate mofetil in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis: a prospective pharmacokinetics and clinical study. Clin Exp Immunol 176(2):172–179
Salvador CL, Tøndel C, Rowe AD, Bjerre A, Brun A, Brackman D, Mørkrid L (2019) Estimating glomerular filtration rate in children: evaluation of creatinine- and cystatin C-based equations. Pediatr Nephrol 34(2):301–311
Woillard JB, Bader-Meunier B, Salomon R et al (2014) Pharmacokinetics of mycophenolate mofetil in children with lupus and clinical findings in favour of therapeutic drug monitoring. Br J Clin Pharmacol 78(4):867–876
Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM (2018) Clinical pharmacokinetics in kidney disease: fundamental principles. Clin J Am Soc Nephrol 13(7):1085–1095
de Winter BC, van Gelder T, Glander P et al (2008) Population pharmacokinetics of mycophenolic acid: a comparison between enteric-coated mycophenolate sodium and mycophenolate mofetil in renal transplant recipients. Clin Pharmacokinet 47(12):827–838
Rong Y, Jun H, Kiang TKL (2021) Population pharmacokinetics of mycophenolic acid in paediatric patients. Br J Clin Pharmacol 87(4):1730–1757
Shemesh O, Golbetz H, Kriss JP, Myers BD (1985) Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 28(5):830–838
Kidney Disease: Improving global outcomes KDIGO CKD work group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3(1):1–150. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1
Brou NA, Jacqz-Aigrain E, Zhao W (2015) Cystatin C as a potential biomarker for dosing of renally excreted drugs. Br J Clin Pharmacol 80(1):20–27
Barreto EF, Rule AD, Murad MH et al (2019) Prediction of the renal elimination of drugs with cystatin C vs creatinine: a systematic review. Mayo Clin Proc 94(3):500–514
Downes KJ, Zane NR, Zuppa AF (2020) Effect of Cystatin C on vancomycin clearance estimation in critically Ill children using a population pharmacokinetic modeling approach. Ther Drug Monit 42(6):848–855
Tan SJ, Cockcroft M, Page-Sharp M, Arendts G, Davis TME, Moore BR, Batty KT, Salman S, Manning L (2020) Population pharmacokinetic study of ceftriaxone in elderly patients, using cystatin C-based estimates of renal function to account for frailty. Antimicrob Agents Chemother 64(10):e00874-e920
Björk J, Nyman U, Berg U et al (2019) Validation of standardized creatinine and cystatin C GFR estimating equations in a large multicentre European cohort of children. Pediatr Nephrol 34(6):1087–1098
Couser WG, Johnson RJ (2015) What is myeloperoxidase doing in ANCA-associated glomerulonephritis? Kidney Int 88(5):938–940
Shah RR, Smith RL (2015) Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab Dispos 43(3):400–410
Lamba V, Sangkuhl K, Sanghavi K, Fish A, Altman RB, Klein TE (2014) PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics 24(1):73–79
Knights KM, Rowland A, Miners JO (2013) Renal drug metabolism in humans: the potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT). Br J Clin Pharmacol 76(4):587–602
de Jong LM, Jiskoot W, Swen JJ, Manson ML (2020) Distinct effects of inflammation on cytochrome P450 regulation and drug metabolism: lessons from experimental models and a potential role for pharmacogenetics. Genes (Basel) 11(12):1509
Richardson TA, Sherman M, Kalman D, Morgan ET (2006) Expression of UDP-glucuronosyltransferase isoform mRNAs during inflammation and infection in mouse liver and kidney. Drug Metab Dispos 34(3):351–353
Kawase A, Norikane S, Okada A, Adachi M, Kato Y, Iwaki M (2014) Distinct alterations in ATP-binding cassette transporter expression in liver, kidney, small intestine, and brain in adjuvant-induced arthritic rats. J Pharm Sci 103(8):2556–2564
Evers R, Piquette-Miller M, Polli JW et al (2018) Disease-associated changes in drug transporters may impact the pharmacokinetics and/or toxicity of drugs: a white paper from the international transporter consortium. Clin Pharmacol Ther 104(5):900–915
Udy AA, Roberts JA, Lipman J (2011) Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol 7(9):539–543
Barau C, Barrail-Tran A, Hemerziu B, Habes D, Taburet AM, Debray D, Furlan V (2011) Optimization of the dosing regimen of mycophenolate mofetil in pediatric liver transplant recipients. Liver Transpl 17(10):1152–1158
Barau C, Mellos A, Chhun S, Lacaille F, Furlan V (2017) Pharmacokinetics of mycophenolic acid and dose optimization in children after intestinal transplantation. Ther Drug Monit 39(1):37–42
Weber LT, Hoecker B, Armstrong VW, Oellerich M, Tönshoff B (2008) Long-term pharmacokinetics of mycophenolic acid in pediatric renal transplant recipients over 3 years posttransplant. Ther Drug Monit 30(5):570–575
Acknowledgements
The authors thank Mr. Yong-chao Fu (Tri-I Biotech Ltd., Shanghai, China) and Mr. Wu Liang (Changsha VALS Technology Co.Ltd., Hunan, China) for their guidance in model building.
Funding
This work was supported by the National Natural Science Foundation [grant number 81874325]; Scientific Research Project of Science and Technology Commission of Shanghai Municipality [grant numbers 18DZ1910604, 19XD1400900, 19DZ1910703].
Author information
Authors and Affiliations
Contributions
ZWL designed the study, developed the models and wrote the manuscript. YDH were involved in patient data collection and acquisition. HX and ZPL provided support for the research and reviewed the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee from Children’s Hospital of Fudan University (No. 2020–486).
Consent for publication
Not applicable.
Conflict of interest
Authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Huang, Y., Xu, H. et al. Population pharmacokinetic and dose optimization of mycophenolic acid in children with anti-neutrophilic cytoplasmic antibody-associated nephritis. Eur J Clin Pharmacol 78, 831–838 (2022). https://doi.org/10.1007/s00228-021-03265-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03265-z