Abstract
Purpose
To develop and validate a population pharmacokinetic (PPK) model of valproic acid (VPA) in adult Chinese patients with bipolar disorder, and provide guidance for individualized therapy in this population.
Methods
A total of 1104 serum concentrations from 272 patients were collected in this study. The data analysis was performed using a nonlinear mixed-effects modeling approach. Covariates included demographic parameters, biological characteristics, and concomitant medications. Bootstrap validation (1000 runs), normalized prediction distribution error (NPDE), and external validation of 50 patients were employed to evaluate the final model.
Results
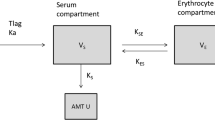
A one-compartment model with first-order absorption and elimination was developed for VPA extended-release tablets. VPA clearance was significantly influenced by three variables: sex (12% higher in male patients), daily dose (increasing with the 0.13 exponent), and body weight (increasing with the 0.56 exponent). Typical values for the absorption rate constant (Ka), apparent clearance (CL/F), and apparent distribution volume (V/F) for a female patient weighing 70 kg administered VPA 1000 mg/day were 0.18 h−1, 0.46 L/h, and 12.84 L, respectively. The results of model evaluation indicated a good stable and precise performance of the final model.
Conclusions
A qualified PPK model of VPA was developed in Chinese patients with bipolar disorder. This model could be used as a suitable tool for the personalization of VPA dosing for bipolar patients.







Similar content being viewed by others
References
McIntyre RS, Berk M, Brietzke E, Goldstein BI, Lopez-Jaramillo C, Kessing LV, Malhi GS, Nierenberg AA, Rosenblat JD, Majeed A, Vieta E, Vinberg M, Young AH, Mansur RB (2020) Bipolar disorders. Lancet 396(10265):1841–1856. https://doi.org/10.1016/S0140-6736(20)31544-0
Rantala MJ, Luoto S, Borraz-Leon JI, Krams I (2021) Bipolar disorder: An evolutionary psychoneuroimmunological approach. Neurosci Biobehav Rev 122:28–37. https://doi.org/10.1016/j.neubiorev.2020.12.031
Vigo D, Thornicroft G, Atun R (2016) Estimating the true global burden of mental illness. Lancet Psychiatry 3(2):171–178. https://doi.org/10.1016/S2215-0366(15)00505-2
Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z (2011) Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 68(3):241–251. https://doi.org/10.1001/archgenpsychiatry.2011.12
Bowden CL (2003) Valproate. Bipolar Disord 5(3):189–202. https://doi.org/10.1034/j.1399-5618.2003.00031.x
Fleming J, Chetty M (2006) Therapeutic monitoring of valproate in psychiatry: how far have we progressed? Clin Neuropharmacol 29(6):350–360. https://doi.org/10.1097/01.WNF.0000228209.69524.E8
Baldessarini RJ, Tondo L, Vazquez GH (2019) Pharmacological treatment of adult bipolar disorder. Mol Psychiatry 24(2):198–217. https://doi.org/10.1038/s41380-018-0044-2
Macritchie K, Geddes JR, Scott J, Haslam D, de Lima M, Goodwin G (2003) Valproate for acute mood episodes in bipolar disorder. Cochrane Database Syst Rev 1:D4052. https://doi.org/10.1002/14651858.CD004052
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, Sharma V, Goldstein BI, Rej S, Beaulieu S, Alda M, MacQueen G, Milev RV, Ravindran A, O’Donovan C, McIntosh D, Lam RW, Vazquez G, Kapczinski F, McIntyre RS, Kozicky J, Kanba S, Lafer B, Suppes T, Calabrese JR, Vieta E, Malhi G, Post RM, Berk M (2018) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 20(2):97–170. https://doi.org/10.1111/bdi.12609
Allen MH, Hirschfeld RM, Wozniak PJ, Baker JD, Bowden CL (2006) Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry 163(2):272–275. https://doi.org/10.1176/appi.ajp.163.2.272
Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, Eckermann G, Egberts K, Gerlach M, Greiner C, Grunder G, Haen E, Havemann-Reinecke U, Hefner G, Helmer R, Janssen G, Jaquenoud E, Laux G, Messer T, Mossner R, Muller MJ, Paulzen M, Pfuhlmann B, Riederer P, Saria A, Schoppek B, Schoretsanitis G, Schwarz M, Gracia MS, Stegmann B, Steimer W, Stingl JC, Uhr M, Ulrich S, Unterecker S, Waschgler R, Zernig G, Zurek G, Baumann P (2018) Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51(1–02):9–62. https://doi.org/10.1055/s-0043-116492
Lin WW, Jiao Z, Wang CL, Wang HY, Ma CL, Huang PF, Guo XZ, Liu YW (2015) Population pharmacokinetics of valproic acid in adult Chinese epileptic patients and its application in an individualized dosage regimen. Ther Drug Monit 37(1):76–83. https://doi.org/10.1097/FTD.0000000000000100
Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HJ, Chou JC, Keck PJ, Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ (2000) A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 57(5): 481–489. https://doi.org/10.1001/archpsyc.57.5.481
Bowden CL, Janicak PG, Orsulak P, Swann AC, Davis JM, Calabrese JR, Goodnick P, Small JG, Rush AJ, Kimmel SE, Risch SC, Morris DD (1996) Relation of serum valproate concentration to response in mania. Am J Psychiatry 153(6):765–770. https://doi.org/10.1176/ajp.153.6.765
Kiang TK, Sherwin CM, Spigarelli MG, Ensom MH (2012) Fundamentals of population pharmacokinetic modelling: modelling and software. Clin Pharmacokinet 51(8):515–525. https://doi.org/10.2165/11634080-000000000-00000
Abdulla A, Rogouti O, Hunfeld N, Endeman H, Dijkstra A, van Gelder T, Muller AE, de Winter B, Koch B (2020) Population pharmacokinetics and target attainment of ciprofloxacin in critically ill patients. Eur J Clin Pharmacol 76(7):957–967. https://doi.org/10.1007/s00228-020-02873-5
Yukawa E, To H, Ohdo S, Higuchi S, Aoyama T (1997) Population-based investigation of valproic acid relative clearance using nonlinear mixed effects modeling: influence of drug-drug interaction and patient characteristics. J Clin Pharmacol 37(12):1160–1167. https://doi.org/10.1002/j.1552-4604.1997.tb04301.x
Blanco-Serrano B, Otero MJ, Santos-Buelga D, García-Sánchez MJ, Serrano J, Domínguez-Gil A (1999) Population estimation of valproic acid clearance in adult patients using routine clinical pharmacokinetic data. Biopharm Drug Dispos 20(5):233–240. https://doi.org/10.1002/(sici)1099-081x(199907)20:5%3c233::aid-bdd179%3e3.0.co;2-5
Yukawa E, Nonaka T, Yukawa M, Higuchi S, Kuroda T, Goto Y (2003) Pharmacoepidemiologic investigation of a clonazepam-valproic acid interaction by mixed effect modeling using routine clinical pharmacokinetic data in Japanese patients. J Clin Pharm Ther 28(6):497–504. https://doi.org/10.1046/j.1365-2710.2003.00528.x
Jankovic SM, Milovanovic JR (2007) Pharmacokinetic modeling of valproate from clinical data in Serbian epileptic patients. Methods Find Exp Clin Pharmacol 29(10):673–679. https://doi.org/10.1358/mf.2007.29.10.1116313
Vucićević K, Miljković B, Pokrajac M, Prostran M, Martinović Z, Grabnar I (2009) The influence of drug-drug interaction and patients’ characteristics on valproic acid’s clearance in adults with epilepsy using nonlinear mixed effects modeling. Eur J Pharm Sci 38(5):512–518. https://doi.org/10.1016/j.ejps.2009.09.017
Jankovic SM, Milovanovic JR, Jankovic S (2010) Factors influencing valproate pharmacokinetics in children and adults. Int J Clin Pharmacol Ther 48(11):767–775. https://doi.org/10.5414/cpp48767
Ibarra M, Vázquez M, Fagiolino P, Derendorf H (2013) Sex related differences on valproic acid pharmacokinetics after oral single dose. J Pharmacokinet Pharmacodyn 40(4):479–486. https://doi.org/10.1007/s10928-013-9323-3
Ogusu N, Saruwatari J, Nakashima H, Noai M, Nishimura M, Deguchi M, Oniki K, Yasui-Furukori N, Kaneko S, Ishitsu T, Nakagaswa K (2014) Impact of the superoxide dismutase 2 Val16Ala polymorphism on the relationship between valproic acid exposure and elevation of γ-glutamyltransferase in patients with epilepsy: a population pharmacokinetic-pharmacodynamic analysis. PLoS ONE 9(11):e111066. https://doi.org/10.1371/journal.pone.0111066
Alqahtani S, Alandas N, Alsultan A (2019) Estimation of apparent clearance of valproic acid in adult Saudi patients. Int J Clin Pharm 41(4):1056–1061. https://doi.org/10.1007/s11096-019-00864-w
Brendel K, Comets E, Laffont C, Laveille C, Mentre F (2006) Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23(9):2036–2049. https://doi.org/10.1007/s11095-006-9067-5
Comets E, Brendel K, Mentre F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90(2):154–166. https://doi.org/10.1016/j.cmpb.2007.12.002
Roberts JA, Kirkpatrick CM, Lipman J (2011) Monte Carlo simulations: maximizing antibiotic pharmacokinetic data to optimize clinical practice for critically ill patients. J Antimicrob Chemother 66(2):227–231. https://doi.org/10.1093/jac/dkq449
Jiao Z, Zhong MK, Shi XJ, Hu M, Zhang JH (2003) Population pharmacokinetics of carbamazepine in Chinese epilepsy patients. Ther Drug Monit 25(3):279–286. https://doi.org/10.1097/00007691-200306000-00005
Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, Holford N, Kimura T, Lo YL, Peris JE, Thomson A, van den Anker JN, Fakhoury M, Jacqz-Aigrain E (2013) External Evaluation of Population Pharmacokinetic Models of Vancomycin in Neonates: The transferability of published models to different clinical settings. Br J Clin Pharmacol 75(4):1068–1080. https://doi.org/10.1111/j.1365-2125.2012.04406.x
Zhao CY, Jiao Z, Mao JJ, Qiu XY (2016) External evaluation of published population pharmacokinetic models of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol 81(5):891–907. https://doi.org/10.1111/bcp.12830
Birnbaum AK, Ahn JE, Brundage RC, Hardie NA, Conway JM, Leppik IE (2007) Population pharmacokinetics of valproic acid concentrations in elderly nursing home residents. Ther Drug Monit 29(5):571–575. https://doi.org/10.1097/FTD.0b013e31811f3296
Methaneethorn J (2017) Population Pharmacokinetics of Valproic Acid in Patients with Mania: Implication for Individualized Dosing Regimens. Clin Ther 39(6):1171–1181. https://doi.org/10.1016/j.clinthera.2017.04.005
Guo J, Huo Y, Li F, Li Y, Guo Z, Han H, Zhou Y (2020) Impact of gender, albumin, and CYP2C19 polymorphisms on valproic acid in Chinese patients: a population pharmacokinetic model. J Int Med Res 48(8):1220751833. https://doi.org/10.1177/0300060520952281
Mei S, Feng W, Zhu L, Li X, Yu Y, Yang W, Gao B, Wu X, Fang F, Zhao Z (2018) Effect of CYP2C19, UGT1A8, and UGT2B7 on valproic acid clearance in children with epilepsy: a population pharmacokinetic model. Eur J Clin Pharmacol 74(8):1029–1036. https://doi.org/10.1007/s00228-018-2440-6
Methaneethorn J (2018) A systematic review of population pharmacokinetics of valproic acid. Br J Clin Pharmacol 84(5):816–834. https://doi.org/10.1111/bcp.13510
Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, Altman RB, Klein TE (2013) Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics 23(4):236–241. https://doi.org/10.1097/FPC.0b013e32835ea0b2
Kim JY, Cheong HS, Park BL, Kim LH, Namgoong S, Kim JO, Kim HD, Kim YH, Chung MW, Han SY, Shin HD (2014) Comprehensive variant screening of the UGT gene family. Yonsei Med J 55(1):232–239. https://doi.org/10.3349/ymj.2014.55.1.232
Fricke-Galindo I, Céspedes-Garro C, Rodrigues-Soares F, Naranjo ME, Delgado Á, de Andrés F, López-López M, Peñas-Lledó E, LLerena A, (2016) Interethnic variation of CYP2C19 alleles, “predicted” phenotypes and “measured” metabolic phenotypes across world populations. Pharmacogenomics J 16(2):113–123. https://doi.org/10.1038/tpj.2015.70
García-Martín E, Martínez C, Ladero JM, Agúndez JA (2006) Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 10(1):29–40. https://doi.org/10.1007/BF03256440
Dorji PW, Wangchuk S, Boonprasert K, Tarasuk M, Na-Bangchang K (2019) Pharmacogenetic relevant polymorphisms of CYP2C9, CYP2C19, CYP2D6, and CYP3A5 in Bhutanese population. Drug Metab Pers Ther 34(4):/j/dmdi.2019.34.issue-4/dmpt-2019–0020/dmpt-2019–0020.xml. https://doi.org/10.1515/dmpt-2019-0020
Jiang D, Bai X, Zhang Q, Lu W, Wang Y, Li L, Müller M (2009) Effects of CYP2C19 and CYP2C9 genotypes on pharmacokinetic variability of valproic acid in Chinese epileptic patients: nonlinear mixed-effect modeling. Eur J Clin Pharmacol 65(12):1187–1193. https://doi.org/10.1007/s00228-009-0712-x
Du Z, Jiao Y, Shi L (2016) Association of UGT2B7 and UGT1A4 Polymorphisms with Serum Concentration of Antiepileptic Drugs in Children. Med Sci Monit 22:4107–4113. https://doi.org/10.12659/msm.897626
Sun YX, Zhuo WY, Lin H, Peng ZK, Wang HM, Huang HW, Luo YH, Tang FQ (2015) The influence of UGT2B7 genotype on valproic acid pharmacokinetics in Chinese epilepsy patients. Epilepsy Res 114:78–80. https://doi.org/10.1016/j.eplepsyres.2015.04.015
Wang P, Lin XQ, Cai WK, Xu GL, Zhou MD, Yang M, He GH (2018) Effect of UGT2B7 genotypes on plasma concentration of valproic acid: a meta-analysis. Eur J Clin Pharmacol 74(4):433–442. https://doi.org/10.1007/s00228-017-2395-z
Jackson J, McCollum B, Ognibene J, Diaz FJ, de Leon J (2015) Three patients needing high doses of valproic Acid to get therapeutic concentrations. Case Rep Psychiatry 2015:542862. https://doi.org/10.1155/2015/542862
Dasgupta A (2007) Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta 377(1–2):1–13. https://doi.org/10.1016/j.cca.2006.08.026
Xikun W, Haoran L, Weichong D, Xiuling Y, Yiran J, Ying G, Zhiqing Z, Xiujv L (2021) Determination of free valproic acid concentration in 569 clinical samples by LC-MS/MS after HFCF-UF treatment. Ther Drug Monit. Epub ahead of print. https://doi.org/10.1097/FTD.0000000000000903
Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, Leppik IE, Tomson T, Perucca E (2008) Antiepileptic drugs–best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring. ILAE Commission on Therapeutic Strategies Epilepsia 49(7):1239–1276. https://doi.org/10.1111/j.1528-1167.2008.01561.x
Sandson NB, Marcucci C, Bourke DL, Smith-Lamacchia R (2006) An interaction between aspirin and valproate: the relevance of plasma protein displacement drug-drug interactions. Am J Psychiatry 163(11):1891–1896. https://doi.org/10.1176/ajp.2006.163.11.1891
Ruan CJ, de Leon J (2019) Thirty Years of Both Ignorance and Clinical Experience Suggest That Clozapine Intoxication During Co-Occurring Infections and Inflammation May Have Higher Morbidity and Mortality Than Is Currently Believed. Psychosomatics 60(2):221–222. https://doi.org/10.1016/j.psym.2018.07.009
Anderson GD (2008) Gender differences in pharmacological response. Int Rev Neurobiol 83:1–10. https://doi.org/10.1016/S0074-7742(08)00001-9
Court MH (2010) Interindividual variability in hepatic drug glucuronidation: studies into the role of age, sex, enzyme inducers, and genetic polymorphism using the human liver bank as a model system. Drug Metab Rev 42(1):209–224. https://doi.org/10.3109/03602530903209288
Franconi F, Brunelleschi S, Steardo L, Cuomo V (2007) Gender differences in drug responses. Pharmacol Res 55(2):81–95. https://doi.org/10.1016/j.phrs.2006.11.001
Wang L, Wang XD (2002) Pharmacokinetic and pharmacodynamic effects of clonazepam in children with epilepsy treated with valproate: a preliminary study. Ther Drug Monit 24(4):532–536. https://doi.org/10.1097/00007691-200208000-00012
Levy RH, Koch KM (1982) Drug interactions with valproic acid. Drugs 24(6):543–556. https://doi.org/10.2165/00003495-198224060-00004
van Wattum PJ (2001) Valproic acid and risperidone. J Am Acad Child Adolesc Psychiatry 40(8):866–867. https://doi.org/10.1097/00004583-200108000-00003
Bertoldo M (2002) Valproic acid and risperidone. J Am Acad Child Adolesc Psychiatry 41(6):632. https://doi.org/10.1097/00004583-200206000-00002
Sund JK, Aamo T, Spigset O (2003) Valproic acid and risperidone: a drug interaction? J Am Acad Child Adolesc Psychiatry 42(1):1–2. https://doi.org/10.1097/00004583-200301000-00001
Spina E, Pisani F, de Leon J (2016) Clinically significant pharmacokinetic drug interactions of antiepileptic drugs with new antidepressants and new antipsychotics. Pharmacol Res 106:72–86. https://doi.org/10.1016/j.phrs.2016.02.014
Besag FM, Berry D (2006) Interactions between antiepileptic and antipsychotic drugs. Drug Saf 29(2):95–118. https://doi.org/10.2165/00002018-200629020-00001
Berigan T, Harazin J (1999) A sertraline/valproic acid drug interaction: Case Reports. Int J Psychiatry Clin Pract 3(4):287–288. https://doi.org/10.3109/13651509909068397
Cloyd JC, Fischer JH, Kriel RL, Kraus DM (1993) Valproic acid pharmacokinetics in children. IV. Effects of age and antiepileptic drugs on protein binding and intrinsic clearance. Clin Pharmacol Ther 53(1):22–29. https://doi.org/10.1038/clpt.1993.5
Acknowledgements
The authors are grateful to all the patients who participated in this study, and all the medical staff in Beijing Anding Hospital who helped conduct this study.
Funding
This study was supported by Capital’s Funds for Health Improvement and Research (Grant No. 2018–4-2124), 2019 NARSAD Young Investigator Award from the Brain & Behavior Research Foundation (Grant No. 28141), National Natural Science Foundation of China (Grant No. 81801322), Beijing Municipal Administration of Hospitals Incubating Program (Grant No. PX2019070).
Author information
Authors and Affiliations
Contributions
YNZ, CJR, and WG were involved in the concept and design of the study. MXN, SB, QW, YW, FD, and ANL were involved in patient recruitment. CJR conducted the analyses of VPA serum concentrations. YNZ and CJR conducted the statistical analyses. YNZ wrote the first draft of the article manuscript, and all authors contributed to subsequent drafts and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Ethical approval
The protocol for the study was approved by the Research Ethics Committee of Beijing Anding Hospital (ethical code: 201965FS-2).
Informed consent
Written informed consent was obtained from all patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zang, YN., Guo, W., Niu, MX. et al. Population pharmacokinetics of valproic acid in adult Chinese patients with bipolar disorder. Eur J Clin Pharmacol 78, 405–418 (2022). https://doi.org/10.1007/s00228-021-03246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03246-2