Abstract
Purpose
This study aimed to compare the utilization of Alzheimer’s disease (AD) treatments, donepezil, galantamine, rivastigmine, and memantine, in Korea with Australia and other countries with universal health coverage.
Methods
Reimbursement criteria and the patent status of four AD treatments in Korea and Australia were reviewed. The monthly spending and utilization of the treatments were extracted from the national electronic database in Korea and Australia. The defined daily dose per 1000 elderly population per day (DDD/1000e/day) were calculated from July 2008 to June 2019. Annual cost trends of Norway and England were compared with Korea and Australia.
Results
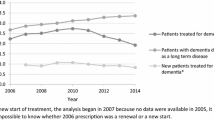
With the highest share of the use of donepezil in both countries, the cost and utilization of AD treatments in Korea increased more rapidly and remained higher than Australia. The cost of AD treatments in Korea increased by 15.5% every year during the study period, while the spending of the same drugs in Australia decreased by 10.5% annually. The utilization in DDD/1000e/day of AD treatments in Korea increased by 18.3% annually compared with 1.4% in Australia. When compared with Norway and England, countries with similar universal health coverage (UHC) system and elderly polupation, the cost of AD treatments in Korea was still higher with the opposite trend from other countries.
Conclusions
Despite the similar UHC systems, there were considerable differences in the post-market utilization of AD treatments in Korea from Australia and other countries. This results can be attributed to differences in re-assessment system, pricing and reimbursement policies, and prescribing culture. This study provides a baseline to explore more comprehensive cross-country studies on rational use of medicines.




Similar content being viewed by others
Data Availability
Data will be made available on responsible request.
References
Alzheimer Society of C (2016) Report summary prevalence and monetary costs of dementia in Canada (2016): a report by the Alzheimer Society of Canada. Health Promot Chronic Dis Prev Can 36(10):231–232
Ross ED, Shah SN, Prodan CI, Monnot M (2006) Changing relative prevalence of Alzheimer disease versus non-Alzheimer disease dementias: have we underestimated the looming dementia epidemic? Dement Geriatr Cogn Disord 22(4):273–277. https://doi.org/10.1159/000095127
Ikejima C, Hisanaga A, Meguro K, Yamada T, Ouma S, Kawamuro Y, Hyouki K, Nakashima K, Wada K, Yamada S (2012) Multicentre population-based dementia prevalence survey in Japan: a preliminary report. Psychogeriatrics 12(2):120–123
Gao LB, Yu XF, Chen Q, Zhou D (2016) Alzheimer’s disease therapeutics: current and future therapies. Minerva Med 107(2):108–113
Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M (2008) Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 148(5):379–397. https://doi.org/10.7326/0003-4819-148-5-200803040-00009
Laver K, Cumming RG, Dyer SM, Agar MR, Anstey KJ, Beattie E, Brodaty H, Broe T, Clemson L, Crotty M, Dietz M, Draper BM, Flicker L, Friel M, Heuzenroeder LM, Koch S, Kurrle S, Nay R, Pond CD, Thompson J, Santalucia Y, Whitehead C, Yates MW (2016) Clinical practice guidelines for dementia in Australia. Med J Aust 204(5):191–193. https://doi.org/10.5694/mja15.01339
Hahn S-J, Paik N-J (2015) Pharmacological Treatment of Dementia. Brain NeuroRehab 8(1):19–23
Lee H-J (2018) The Composition of Pharmaceutical Expenditure in National Health Insurance and Implications for Reasonable Spending. Health Policy Manag 28(4):360–368. https://doi.org/10.4332/KJHPA.2018.28.4.360
Australian Government Department of Health (2019) Post-Market Reviews of Pharmaceutical Benefits Scheme Subsidised Medicines. Pharmaceutical Benefits Scheme (PBS). http://www.pbs.gov.au/info/reviews/subsidised-medicines-reviews. Accessed 12 Dec 2019
Korean Statistical Information Service (KOSIS) (2019) Population in Old Ages. KOSIS. http://kosis.kr/eng/index/index.do. Accessed 12 Dec 2019
Australian Bureau of Statistics (2018) Table 59. Estimated resident population by single year of age. Australia, Australian Bureau of Statistics https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Mar%202019?OpenDocument. Accessed 12 Dec 2019
Ministry of Health and Welfare, National Institute of Dementia (2019) Korean Dementia Observatory 2018. National Institute of Dementia. https://www.nid.or.kr/main/main.aspx. Accessed 19 Dec 2019
Dementia Australia (2018) Dementia Prevalence Data 2018-2058. University of Canberra. https://www.dementia.org.au/information/statistics/prevalence-data. Accessed 20 Oct 2019
Statistics Norway (2020) 07459: Population, by sex and one-year age groups (M) 1986 - 2020. Statistics Norway. https://www.ssb.no/en/statbank/table/07459/. Accessed 07 Oct 2020
Statistics OfN (2019) Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid-2019. Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2019estimates. Accessed 7 Oct 2020
Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, Wittenberg R, Adelaja B, Hu B, King D (2014) Dementia UK: -overview.
Norwegian Institute of Public Health (2019) Dementia in Norway. Norwegian Institute of Public Health. https://www.fhi.no/en/op/hin/health-disease/dementia-in-norway/. Accessed 07 Oct 2020
Weise N, Festøy H, Hagen C (2018) PPRI Pharma Profile Norway 2018. PPRI Pharma, Oslo
Kullman D, Palnoch D (2010) PPRI Pharma Profile United Kingdom 2010. PPRI Pharma, London
Kim SY (2018) Past and Future of Drug Treatments for Alzheimer’s Disease. J Korean Neuropsychiatr Assoc 57(1):30–42
Health Insurance Review and Assessment Service (HIRA) (2019) Pharmaceutical Reimbursement and Price List. HIRA. https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030014050000. Accessed 12 Oct 2019
Pharmaceutical Benefits Scheme (PBS) (2019) Schedule of Pharmaceutical Benefits (Volume 1 - General Schedule). PBS. http://www.pbs.gov.au/pbs/publication/schedule/archive#_2018. Accessed 10 Oct 2019
Health Insurance Review and Assessment Service (HIRA) (2018) Pharmaceutical Reimbursement and Price List. HIRA. https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030014050000. Accessed 12 Oct 2019
KIMS (2019) KIMS Pharmaceutical Information Center. KIMS Online. http://www.kimsonline.co.kr/. Accessed 14 Oct 2019
Australian Government Department of Health (2019) Schedule of Pharmaceutical Benefits. Pharmaceutical Benefits Scheme (PBS). http://www.pbs.gov.au/info/publication/schedule/archive. Accessed 8 Oct 2019
Health Insurance Review and Assessment Service (HIRA) (2019) Announcement of Reimbursement Criteria. HIRA. https://www.hira.or.kr/rd/insuadtcrtr/InsuAdtCrtrList.do?pgmid=HIRAA030069000400. Accessed 10 Sep 2019
National Health Insurance Service (NHIS) (2019) Population Coverage. NHIS. https://www.nhis.or.kr/static/html/wbd/g/a/wbdga0403.html. Accessed 17 Dec 2019
Norwegian Institute of Public Health Statistics from the Norwegian Prescription Database. NIPH. http://www.norpd.no/. Accessed 12 September 2020
Prescription Cost Analysis - England (2019) NHSBSA. https://www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england. Accessed 12 Sept 2020
World Health Organization Collaborating Centre for Drug Statistics Methodology (2018) Guidelines for ATC classification and DDD assignment 2019. Oslo, Norway
Organisation for Economic Co-operation and Development (OECD) (2020) Purchasing power parities (PPP) OECD. https://data.oecd.org/conversion/exchange-rates.htm. Accessed 25th March 2020
Australian Government Department of Health (2018) Schedule of Pharmaceutical Benefits (Volume 1 - General Schedule). Pharmaceutical Benefits Scheme (PBS). http://www.pbs.gov.au/pbs/publication/schedule/archive#_2018. Accessed 10 Oct 2019
Lee DW, Seong S-J (2018) Korean national dementia plans: from 1st to 3rd. J Kor Med Assoc 61(5):298–303
Lee M-S (2019) Preparation and Measures for Elderly with Dementia in Korea: Focus on National Strategies and Action Plan against Dementia. J Agric Med Commun Health 44(1):11–27
Ministry of Health and Welfare (2019) Voucher Program for Dementia Treatments. Ministry of Health and Welfare. http://www.bokjiro.go.kr/welInfo/retrieveWelInfoBoxList.do. Accessed 20 Dec 2019
Kim H-A, Shin J-Y, Kim M-H, Park B-J (2014) Prevalence and predictors of polypharmacy among Korean elderly. PLoS One 9(6):e98043
Page AT, Falster MO, Litchfield M, Pearson SA, Etherton-Beer C (2019) Polypharmacy among older Australians, 2006–2017: a population-based study. Med J Aust 211:71–75
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL (2015) Trends in prescription drug use among adults in the United States from 1999-2012. Jama 314(17):1818–1830
Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T (2015) The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med 13(1):74. https://doi.org/10.1186/s12916-015-0322-7
Australian Government Department of Health (2019) What medicines does the government subsidise? Pharmaceutical Benefits Scheme (PBS). http://www.pbs.gov.au/info/about-the-pbs#What_medicines_does_the_government_subsidise. Accessed 19 Dec 2019
Centre for Health Economics Monash University, University of South Australia, Department of Health and Ageing, Ragg Ahmed(2012) Post Market Review Pharmaceutical Benefits Scheme anti-dementia medicines to treat Alzheimer Disease. Pharmaceutical Benefits Scheme (PBS). http://www.pbs.gov.au/reviews/anti-dementia-drugs-files/anti-dementia-report-summary.pdf. Accessed 12 Aug 2019
Australian Government Department of Health (2012) Review of Pharmaceutical Benefits Scheme Anti-dementia Drugs to Treat Alzheimers Disease. Pharmaceutical Benefits Scheme (PBS). http://www.pbs.gov.au/info/reviews/anti-dementia-drugs. Accessed 10 Aug 2019
Acknowledgments
Author Lisa Kalisch Ellett is supported by a National Health and Medical Research Council and Australian Research Council (NHMRC-ARC) Dementia Research Development Fellowship (Grant identification number APP1101788).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HJL and EER conceived of the presented idea. HJL and JHL performed data collecting, cleaning and analysis and LKE verified the analytical methods. HJL performed literature review. HJL and LKE drafted the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
HJL and JHL were employees of National Health Insurance Service when this research was done. Other authors declare that they have no competing interests.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 102 kb)
Rights and permissions
About this article
Cite this article
Lee, HJ., Roughead, E.E., Han, E. et al. Post-market utilization patterns of Alzheimer’s disease treatments in South Korea: comparison with countries with universal health coverage. Eur J Clin Pharmacol 77, 921–929 (2021). https://doi.org/10.1007/s00228-020-03065-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-03065-x