Abstract
Purpose
The currently recommended dosages of atezolizumab for patients with non-small cell lung cancer (NSCLC) and urothelial carcinoma (UC) is 840 mg every 2 weeks, 1200 mg every 3 weeks (q3w), and 1680 mg every 4 weeks (q4w). However, it has been argued that these dosages may not be optimal. This study aimed to explore the feasibility of extended dosing regimens by population pharmacokinetics (PK) simulations and exposure–response (E-R) relationships.
Methods
All simulations were conducted based on the established population PK and E-R model for safety (i.e., adverse events of special interest, AESI) and efficacy (i.e., objective response rate, ORR) for patients with NSCLC or UC. The PK, AESI, and ORR profiles of the following dosing regimens were simulated: (i) 840 mg q4w, (ii) 1200 mg every 6 weeks (q6w), and (iii) 1680 mg q8w. These regimens were compared with those of the 1200 mg q3w standard regimen.
Results
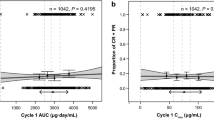
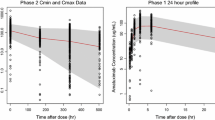
The simulation revealed that the ranking of efficacy for different extended dosing regimens were 1680 mg q8w ≅ 1200 mg q3w ≅ 1200 mg q6w > 840 mg q4w based on the predicted probability of ORR in patients with NSCLC and UC, and this ranking order was similar to that of the safety outcome of the AESI. The minimum serum concentration at steady-state (Cmin,ss) values for all dosing regimens was all higher than the target effective concentration of 6 μg/mL.
Conclusion
The findings from this simulation suggest that extended dosing regimens are unlikely to significantly impair clinical outcomes and may provide more therapeutic benefits to patients in terms of safety.


Similar content being viewed by others
Data availability
All data was generated from the published model.
References
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387(10030):1837–1846. https://doi.org/10.1016/s0140-6736(16)00587-0
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387(10031):1909–1920. https://doi.org/10.1016/s0140-6736(16)00561-4
Morrissey KM, Marchand M, Patel H, Zhang R, Wu B, Phyllis Chan H, Mecke A, Girish S, Jin JY, Winter HR, Bruno R (2019) Alternative dosing regimens for atezolizumab: an example of model-informed drug development in the postmarketing setting. Cancer Chemother Pharmacol 84(6):1257–1267. https://doi.org/10.1007/s00280-019-03954-8
Freshwater T, Kondic A, Ahamadi M, Li CH, de Greef R, de Alwis D, Stone JA (2017) Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer 5:43. https://doi.org/10.1186/s40425-017-0242-5
Long GV, Tykodi SS, Schneider JG, Garbe C, Gravis G, Rashford M, Agrawal S, Grigoryeva E, Bello A, Roy A, Rollin L, Zhao X (2018) Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol 29(11):2208–2213. https://doi.org/10.1093/annonc/mdy408
Novakovic AM, Wilkins JJ, Dai H, Wade JR, Neuteboom B, Brar S, Bello CL, Girard P, Khandelwal A (2019) Changing body weight-based dosing to a flat dose for avelumab in metastatic Merkel cell and advanced urothelial carcinoma. Clin Pharmacol Ther 107:588–596. https://doi.org/10.1002/cpt.1645
Staab A, Rook E, Maliepaard M, Aarons L, Benson C (2013) Modeling and simulation in clinical pharmacology and dose finding. CPT Pharmacometrics Syst Pharmacol 2:e29. https://doi.org/10.1038/psp.2013.5
TGA. Extract from the Clinical Evaluation Report for Atezolizumab. 2017. https://www.tga.gov.au/sites/default/files/auspar-atezolizumab-180903-cer.pdf. Accessed date: 2 January 2020.
Stroh M, Winter H, Marchand M, Claret L, Eppler S, Ruppel J, Abidoye O, Teng SL, Lin WT, Dayog S, Bruno R, Jin J, Girish S (2017) Clinical pharmacokinetics and pharmacodynamics of atezolizumab in metastatic urothelial carcinoma. Clin Pharmacol Ther 102(2):305–312. https://doi.org/10.1002/cpt.587
Goldstein DA, Ratain MJ (2019) Alternative dosing regimens for atezolizumab: right dose, wrong frequency. Cancer Chemother Pharmacol 84(6):1153–1155. https://doi.org/10.1007/s00280-019-03971-7
Deng R, Bumbaca D, Pastuskovas CV, Boswell CA, West D, Cowan KJ, Chiu H, McBride J, Johnson C, Xin Y, Koeppen H, Leabman M, Iyer S (2016) Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. mAbs 8(3):593–603. https://doi.org/10.1080/19420862.2015.1136043
FDA. Clinical pharmacology biopharmaceutics review(s) for atezolizumab. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000MedR.pdf, Accessed date: 2 January 2020.
Ratain MJ, Goldstein DA (2018) Time is money: optimizing the scheduling of nivolumab. J Clin Oncol. https://doi.org/10.1200/jco.18.00045
Renner A, Burotto M, Rojas C (2019) Immune checkpoint inhibitor dosing: can we go lower without compromising clinical efficacy? J Global Oncol (5):1–5. https://doi.org/10.1200/jgo.19.00142
Ogungbenro K, Patel A, Saunders M, Clark J, Duncombe R (2019) An evaluation of cetuximab dosing strategies using pharmacokinetics and cost analysis. J Pharm Pharmacol 71(8):1222–1230. https://doi.org/10.1111/jphp.13108
Peer CJ, Goldstein DA, Goodell JC, Nguyen R, Figg WD, Ratain MJ (2020) Opportunities for using in silico-based extended dosing regimens for monoclonal antibody immune checkpoint inhibitors. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.14369
FDA. Exposure-Response Relationships-Study Design, Data Analysis, and Regulatory Applications. 2003. https://www.fda.gov/media/71277/download. Accessed date: 24 June 2020.
FDA. Clinical trial endpoints for the approval of cancer drugs and biologics. 2018. https://www.fda.gov/media/71195/download. Accessed date: 2 January 2020.
Fleming TR (2005) Objective response rate as a surrogate end point: a commentary. J Clin Oncol 23(22):4845–4846. https://doi.org/10.1200/jco.2005.92.008
Ito K, Miura S, Sakaguchi T, Murotani K, Horita N, Akamatsu H, Uemura K, Morita S, Yamamoto N (2019) The impact of high PD-L1 expression on the surrogate endpoints and clinical outcomes of anti-PD-1/PD-L1 antibodies in non-small cell lung cancer. Lung Cancer (Amsterdam) 128:113–119. https://doi.org/10.1016/j.lungcan.2018.12.023
Acknowledgments
The authors would like to thank Dr. Min-Shung Lin for his careful reading of manuscript and helpful suggestions.
Author information
Authors and Affiliations
Contributions
LFH wrote the manuscript and designed research; CCH analyzed data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Disclaimer
The views expressed in this article are the author’s personal opinions and not necessarily recommendations of the Taiwan CDE.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chou, CH., Hsu, LF. Model-based simulation to support the extended dosing regimens of atezolizumab. Eur J Clin Pharmacol 77, 87–93 (2021). https://doi.org/10.1007/s00228-020-02980-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02980-3