Abstract
Objective
We previously investigated the pharmacokinetic and pharmacodynamic (PK/PD) parameters of routine linezolid infusions (1 h) in patients with external ventricular drains (EVD). The aim of the study was to determine whether extended linezolid infusions (200 mg/h for 3 h) were more efficacious than short linezolid infusions (600 mg/h for 1 h).
Methods
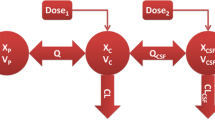
We collected cerebrospinal fluid (CSF) and plasma samples from 10 patients who received linezolid infusions after cerebral hemorrhage surgery with EVDs. Linezolid concentrations were measured by high-performance liquid chromatography (HPLC). A Monte Carlo simulation was used to measure the probability of target attainments (PTA) and the PK/PD indexes at four minimum inhibitory concentrations (MIC).
Results
When the same dose (600 mg) was given as an extended infusion (3 h), linezolid reached its maximum concentrations in the plasma and CSF at 3.00 h and 4.40 h, respectively. The mean penetration of linezolid in CSF was 41.31%. Using the parameter of AUC0–24 h/MIC ≥ 100, the plasma PTA provided good coverage at > 90% when MIC was ≤ 1 μg/mL, while the values were 0 in CSF. Using the parameter %T (time) > MIC ≥ 85%, the PTA in both the plasma and CSF provided good coverage when MIC ≤ 2 μg/mL. Compared with routine infusions, prolonged infusion times (3 h) showed increased PTA of linezolid.
Conclusions
Prolonged infusion times increased the concentration of linezolid in the plasma, leading to improved therapeutic outcomes. However, this improvement did not exist in CSF. Lastly, the PK/PD indicator AUC/MIC ≥ 100 may be used to achieve improved outcomes in patients with critical infections.


Similar content being viewed by others
References
Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L (2006) Risk factors for adult nosocomial meningitis after craniotomy role of antibiotic prophylaxis. Neurosurgery 59:126–133
Lele AV, Hoefnagel AL, Schloemerkemper N, Wyler DA, Chaikittisilpa N, Vavilala MS, Naik BI, Williams JH, Venkat Raghavan L, Koerner IP (2017) Perioperative management of adult patients with external ventricular and lumbar drains: guidelines from the Society for Neuroscience in Anesthesiology and Critical Care. J Neurosurg Anesthesiol 29:191–210
Zhang Y, Zhang J, Chen Y, Yu J, Cao G, Wu X, Chen M, Wu J, Zhao X (2017) Evaluation of meropenem penetration into cerebrospinal fluid in patients with meningitis after neurosurgery. World Neurosurg 98:525–531
Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr (2008) Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 62:688–700
Hagel S, Bruns T, Pletz MW, Engel C, Kalff R, Ewald C (2014) External ventricular drain infections: risk factors and outcome. Interdiscip Perspect Infect Dis 2014:708531
Eymann R, Chehab S, Strowitzki M, Steudel WI, Kiefer M (2008) Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr 1:444–450
Lyke KE, Obasanjo OO, Williams MA, O'Brien M, Chotani R, Perl TM (2001) Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis 33:2028–2033
Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Michael Scheld W, van de Beek D, Bleck TP, Garton HJ, Zunt JR (2017) 2017 Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis 64:701–706
Chen K, Wu Y, Wang Q, Wang J, Li X, Zhao Z, Zhou J (2015) The methodology and pharmacokinetics study of intraventricular administration of vancomycin in patients with intracranial infections after craniotomy. J Crit Care 30(218):e211–e215
Lutsar I, Friedland IR (2000) Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet 39:335–343
Rageh AH, Atia NN, Abdel-Rahman HM (2018) Application of salting-out thin layer chromatography in computational prediction of minimum inhibitory concentration and blood-brain barrier penetration of some selected fluoroquinolones. J Pharm Biomed Anal 159:363–373
Humphreys H, Jenks P, Wilson J, Weston V, Bayston R, Waterhouse C, Moore A, Healthcare Infection Society Working Party on Neurosurgical Infections (2017) Surveillance of infection associated with external ventricular drains: proposed methodology and results from a pilot study. J Hosp Infect 95:154–160
Villani P, Regazzi MB, Marubbi F, Viale P, Pagani L, Cristini F, Cadeo B, Carosi G, Bergomi R (2002) Cerebrospinal fluid linezolid concentrations in postneurosurgical central nervous system infections. Antimicrob Agents Chemother 46:936–937
Zahedi Bialvaei A, Rahbar M, Yousefi M, Asgharzadeh M, Samadi Kafil H (2017) Linezolid: a promising option in the treatment of Gram-positives. J Antimicrob Chemother 72:354–364
Tsuji Y, Hiraki Y, Matsumoto K, Mizoguchi A, Sadoh S, Kobayashi T, Sakamoto S, Morita K, Yukawa E, Kamimura H, Karube Y (2012) Evaluation of the pharmacokinetics of linezolid in an obese Japanese patient. Scand J Infect Dis 44:626–629
Cunha BA (2006) Antimicrobial therapy of multidrug-resistant Streptococcus pneumoniae, vancomycin-resistant enterococci, and methicillin-resistant Staphylococcus aureus. Med Clin North Am 90:1165–1182
Nukui Y, Hatakeyama S, Okamoto K, Yamamoto T, Hisaka A, Suzuki H, Yata N, Yotsuyanagi H, Moriya K (2013) High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother 68:2128–2133
Ippolito JA, Kanyo ZF, Wang D, Franceschi FJ, Moore PB, Steitz TA, Duffy EM (2008) Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J Med Chem 51:3353–3356
De Vriese AS, Coster RV, Smet J, Seneca S, Lovering A, Van Haute LL, Vanopdenbosch LJ, Martin JJ, Groote CC, Vandecasteele S, Boelaert JR (2006) Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis 42:1111–1117
Barrasa H, Soraluce A, Isla A, Martín A, Maynar J, Canut A, Sánchez-Izquierdo JA, Rodríguez-Gascón A (2019) Pharmacokinetics of linezolid in critically ill patients on continuous renal replacement therapy: influence of residual renal function on PK/PD target attainment. J Crit Care 50:69–76
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF (2011) Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:e18–e55
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J (2012) Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629
Hashemian SMR, Farhadi T, Ganjparvar M (2018) Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther 12:1759–1767
Wu X, Tang Y, Zhang X, Wu C, Kong L (2018) Pharmacokinetics and pharmacodynamics of linezolid in plasma/cerebrospinal fluid in patients with cerebral hemorrhage after lateral ventricular drainage by Monte Carlo simulation. Drug Des Devel Ther 12:1679–1684
Zhu LL, Zhou Q (2018) Optimal infusion rate in antimicrobial therapy explosion of evidence in the last five years. Infect Drug Resist 11:1105–1117
Cai Y, Bai N, Liu X, Liang B, Wang J, Wang R (2015) Pharmacokinetic/pharmacodynamic research on three different infusion time regimens of linezolid in healthy Chinese volunteers. Int J Clin Pharmacol Ther 53:765–771
Fernandes G, Salgado HRN, Santos JLD (2019) A critical review of HPLC-based analytical methods for quantification of linezolid. Crit Rev Anal Chem:1–16
Craig WA (2003) Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin N Am 17:479–501
Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ (2003) Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet 42:1411–1423
Andes D, van Ogtrop ML, Peng J, Craig WA (2002) In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 46:3484–3489
Stalker DJ, Jungbluth GL (2003) Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet 42:1129–1140
Di Paolo A, Malacarne P, Guidotti E, Danesi R, Del Tacca M (2010) Pharmacological issues of linezolid: an updated critical review. Clin Pharmacokinet 49:439–447
Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I (1997) Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670–1673
Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR (1999) Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med 340:493–501
Colin P, Eleveld DJ, Jonckheere S, Van Bocxlaer J, De Waele J, Vermeulen A (2016) What about confidence intervals? A word of caution when interpreting PTA simulations. J Antimicrob Chemother 71:2502–2508
Caffrey AR, Morrill HJ, Puzniak LA, Laplante KL (2014) Comparative effectiveness of linezolid and vancomycin among a national veterans affairs cohort with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy 34:473–480
Fu J, Ye X, Chen C, Chen S (2013) The efficacy and safety of linezolid and glycopeptides in the treatment of Staphylococcus aureus infections. PLoS One 8:e58240
Vardakas KZ, Kioumis I, Falagas ME (2009) Association of pharmacokinetic and pharmacodynamic aspects of linezolid with infection outcome. Curr Drug Metab 10:2–12
Falagas ME, Vardakas KZ (2008) Benefit-risk assessment of linezolid for serious gram-positive bacterial infections. Drug Saf 31:753–768
Luque S, Grau S, Alvarez-Lerma F, Ferrandez O, Campillo N, Horcajada JP, Basas M, Lipman J, Roberts JA (2014) Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int J Antimicrob Agents 44:409–415
Viaggi B, Di Paolo A, Danesi R, Polillo M, Ciofi L, Del Tacca M, Malacarne P (2011) Linezolid in the central nervous system: comparison between cerebrospinal fluid and plasma pharmacokinetics. Scand J Infect Dis 43:721–727
Myrianthefs P, Markantonis SL, Vlachos K, Anagnostaki M, Boutzouka E, Panidis D, Baltopoulos G (2006) Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob Agents Chemother 50:3971–3976
Chant C, Leung A, Friedrich JO (2013) Optimal dosing of antibiotics in critically ill patients by using continuous/extended infusions: a systematic review and meta-analysis. Crit Care 17(6):R279
Funding
This work was funded by the Research Innovation Program for College Graduates of Bengbu Medical College (Byycxz1833), Key Special Project of Translational Medicine Research of Bengbu Medical College (BYTM2019015), and Program of Study Abroad for the Excellent Youth Key Scholars of Education Department of Anhui Province of China (gxgwfx2019030).
Author information
Authors and Affiliations
Contributions
Wenjun Zhao and Lingti Kong contributed equally to this work. Wenjun Zhao, Lingti Kong, and Xiaofei Wu participated in research design, performed the research, and wrote the manuscript. Wenjun Zhao and Chenchen Wu conducted the experiment. All authors contributed towards critically revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
This experiment was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, W., Kong, L., Wu, C. et al. Prolonged infusion of linezolid is associated with improved pharmacokinetic/pharmacodynamic (PK/PD) profiles in patients with external ventricular drains. Eur J Clin Pharmacol 77, 79–86 (2021). https://doi.org/10.1007/s00228-020-02978-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02978-x