Abstract
Purpose
To describe the exposure to drugs used for the treatment of patent ductus arteriosus (PDA) in a large cohort of preterm infants born before 32 weeks of gestation.
Methods
A prospective observational cohort analysis was conducted during 2 years in 28 French level 3 NICU using the same computerized order-entry system. The main outcome was “a medically treated PDA,” defined as exposure to ibuprofen, indomethacin, or paracetamol prescribed with the indication of PDA closure. Secondary outcomes were as follows: time of the first treatment administration; total exposure to furosemide during hospitalization; and rate of PDA refractory to pharmacological closure.
Results
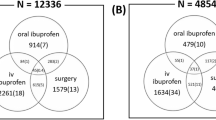
The study cohort consisted of 2614 infants. Among them, 474 (18.1%) received a medical treatment for PDA, with a mean postnatal age at treatment of 4.3 ± 6.6 days. The drug used as a first-line treatment was ibuprofen in 89.5% and paracetamol in 10.5%. One hundred and ninety-five infants (7.4%) had a PDA refractory to pharmacological closure. At the multivariate analysis, factors associated with PDA refractory to pharmacological closure (OR; 95% CI) were as follows: gestational age (GA) (0.81; 0.72–0.90), paracetamol as the first-line treatment (0.32; 0.15–0.68), and pharmacological treatment before 48 h of life (0.63; 0.43–0.94). 24.6% of the study cohort was exposed to furosemide (cumulative dose 6.5 ± 12.6 mg/kg). Variables significantly associated with higher cumulative doses of furosemide were lower GA and ibuprofen treatment (both p < 0.0001).
Conclusion
Drug utilization patterns in infants with PDA vary among centers. Pharmacoepidemiology studies can provide new information on factors associated with PDA refractory to medical treatment.



Similar content being viewed by others
Data availability
All relevant data are within the paper.
References
Dice JE, Bhatia J (2007) Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther 12(3):138–146. https://doi.org/10.5863/1551-6776-12.3.138
Edstedt Bonamy A-K, Gudmundsdottir A, Maier RF, Toome L, Zeitlin J, Bonet M, Fenton A, Hasselager AB, van Heijst A, Gortner L, Milligan D, van Reempts P, Boyle EM, Norman M, and collaborators from the EPICE Research Group (2017) Patent ductus arteriosus treatment in very preterm infants: a European population-based cohort study (EPICE) on variation and outcomes. Neonatology 111(4):367–375. https://doi.org/10.1159/000454798
Group Express. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS) (2010). Acta Paediatr 99(7): 978–992. doi: https://doi.org/10.1111/j.1651-2227.2010.01846
Hamrick SEG, Hansmann G (2010) Patent ductus arteriosus of the preterm infant. Pediatrics 125(5):1020–1030. https://doi.org/10.1542/peds.2009-3506
Sinha B (2013) Controversies in management of patent ductus arteriosus in the preterm infant. J Pulmon Resp Med S 13:007. https://doi.org/10.4172/2161-105X.S13-007
Chaudhary N, Filipov P, Bhutada A, Rastogi S (2016) Controversies in the management of patent ductus arteriosus in preterm infants. J Neonatal Biol 5:238. https://doi.org/10.4172/2167-0897.1000238
Rozé JC, Cambonie G, Marchand-Martin L, Gournay V, Durrmeyer X, Durox M, Storme L, Porcher R, Ancel PY, Hemodynamic EPIPAGE 2 Study Group (2015) Association between early screening for patent ductus arteriosus and in-hospital mortality among extremely preterm infants. JAMA 313(24):2441–2448. https://doi.org/10.1001/jama.2015.6734
Gouyon B, Iacobelli S, Saliba E, Quantin C, Pignolet A, Jacqz-Aigrain E, Gouyon JB (2017) A computer prescribing. Order entry-clinical decision support system designed for neonatal care: results of the ‘preselected prescription’ concept at the bedside. J Clin Pharm Ther 42(1):64–68. https://doi.org/10.1111/jcpt.12474
Gouyon B, Martin-Mons S, Iacobelli S, Razafimahefa H, Kermorvant-Duchemin E, Brat R, Caeymaex L, Couringa Y, Alexandre C, Lafon C, Ramful D, Bonsante F, Binson G, Flamein F, Moussy-Durandy A, di Maio M, Mazeiras G, Girard O, Desbruyeres C, Mourdie J, Escourrou G, Flechelles O, Abasse S, Rosenthal JM, Pages AS, Dorsi M, Karaoui L, ElGellab A, le Bail Dantec F, Yangui MA, Norbert K, Kugbe Y, Lorrain S, Pignolet A, Garnier EM, Lapillonne A, Mitanchez D, Jacqz-Aigrain E, Gouyon JB (2019) Characteristics of prescription in 29 Level 3 Neonatal Wards over a 2-year period (2017-2018). An inventory for future research. PLoS One 14(9):e0222667. https://doi.org/10.1371/journal.pone.0222667
Taketomo CKHJH, Kraus DM (2016) Pediatric and neonatal dosage handbook. Lexicomp. 23th ed. Hudson: Wolters Kluwer Clinical Druf Information
Dani C, Mosca F, Cresi F, Lago P, Lista G, Laforgia N, del Vecchio A, Corvaglia L, Paolillo P, Trevisanuto D, Capasso L, Fanos V, Maffei G, Boni L (2019) Patent ductus arteriosus in preterm infants born at 23–24 weeks’ gestation: should we pay more attention? Early Hum Dev 135:16–22. https://doi.org/10.1016/j.earlhumdev.2019.06.002
El-Khuffash A, Jain A, Corcoran D, Shah PS, Hooper CW, Brown N et al (2014) Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatr Res 76:238–244. https://doi.org/10.1038/pr.2014.82
Evans N (2015) Preterm patent ductus arteriosus: a continuing conundrum for the neonatologist? Semin Fetal Neonatal Med 20(4):272–277. https://doi.org/10.1016/j.siny.2015.03.004
Kluckow M, Jeffery M, Gill A, Evans N (2014) A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 99(2):F99–104. doi: https://doi.org/10.1136/archdischild-2013-304695
Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Smith P (2014) Medication use in the neonatal intensive care unit. Am J Perinatol 31:811–822. https://doi.org/10.1055/s-0033-1361933
Gimpel C, Krause A, Franck P, Krueger M, von Schnakenburg C (2010) Exposure to furosemide as the strongest risk factor for nephrocalcinosis in preterm infants. Pediatr Int 52(1):51–56. https://doi.org/10.1111/j.1442-200X.2009.02886.x
Bell EF, Acarregui MJ (2014) Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 12:CD000503. https://doi.org/10.1002/14651858
Vanhaesebrouck S, Zonnenberg I, Vandervoort P, Bruneel E, Van Hoestenberghe MR, Theyskens C (2007) Conservative treatment for patent ductus arteriosus in the preterm. Archives of Disease in Childhood - Fetal and Neonatal Edition 92 (4):F244-F247
Acknowledgments
We thank all doctors and hospitals who accepted the principle of continuous benchmarking as a way for an improved quality of care: ABASSE Soumeth CH Mayotte, AUTRET Fanny Hôpital Privé St-Joseph Paris, BINSON Guillaume CHU Poitiers, BRAT Roselyne CHR Orléans, BOIZE Philippe CH Pontoise, CAEYMAEX Laurence CHI Créteil, CENERIC Alexandre CHU Caen, DESBRUYERES Céline CH Métropole Savoie, Di MAIO Massimo CHU Nîmes, ELGELLAB Abdellah CH Lens, ESCOURROU Guillaume CHI Montreuil, FLAMEIN Florence CHRU Lille, FLECHELLES Olivier CHU Fort-de-France, GIRARD Olivier CH St-Denis, KARAOUI Leila CH Meaux, KUGBE Yaovi CH Ouest Guyanais, LAFON Catherine CH Arras, LE BAIL DANTEC Florence CH St-Brieuc, MAZEIRAS Gaël CH Côte Basque, MOURDIE Julien CH Le Havre, MOUSSY-DURANDY Amélie CHI Poissy-St-Germain, NORBERT Karine CH Pau, PAGES Anne-Sophie CH Cotentin, RAMFUL Duksha CHU St-Denis de La Réunion, RANDRIANJAFINIMPANANA Heritiana CH Cayenne, RAZAFIMAHEFA Hasinirina CH Sud Francilien, ROSENTHAL Jean-Marc CHU Guadeloupe, and ZIAD Assaf Necker-Enfants Malades.
Funding
This study is part of a European research program FEDER number 2014–34018 (https://ec.europa.eu/regional_policy/en/funding/erdf/) about benchmark of prescription in NICU. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
SI conceptualized the study, participated in the interpretation of the results, and wrote the paper; SL was in charge of the database quality check and formal analyses; BG contributed substantially to the conception of the study; SG participated in the redaction of the study protocol; NL critically reviewed the manuscript draft; JBG participated in the interpretation of the results and critically reviewed the manuscript. FB conceptualized and designed the study, oversaw the data analysis and interpretation, and critically reviewed and revised the manuscript drafts. All authors accept responsibility for the paper as submitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Competing interests: Dr. Beatrice Gouyon (3rd author) was in charge of the B-PEN program (Benchmarking of Prescription in Neonatology) in the research center (CEPOI) from 2012 to 2016. Her employer was the University Hospital (CHU) of La Réunion. Then Dr. B Gouyon left the CEPOI to take the lead of a new start-up (LogipremF comp.) responsible for the development of the prescription software Logipren and its implementation in the NICUs of the B-PEN network. This change of affiliation has been contracted with the University Hospital of La Réunion, after authorization from the “Commission de Déontologie de la Fonction Publique” was obtained. Dr. B Gouyon remained closely involved in the research projects issued from the B-PEN database particularly. All other authors declare that they have no competing interests.
Ethics approval
This study was conducted in accordance with French legislation. As per French law applicable to trials involving human subjects (Jardé Act), a specific approval of an ethics committee is not required for this non-interventional study based on anonymized data of authorized collections (declaration number CNIL: DE-2015-099, DE-2017-410).
Consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript and gave consent for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iacobelli, S., Lorrain, S., Gouyon, B. et al. Drug exposure for PDA closure in France: a prospective, cohort-based, analysis. Eur J Clin Pharmacol 76, 1765–1772 (2020). https://doi.org/10.1007/s00228-020-02974-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02974-1