Abstract
Purpose
The usual recommended dose for gentamicin is 3 to 7 mg/kg/day for patients with a normal renal function while 1.7 mg/kg/day is recommended for patients undergoing chronic haemodialysis. The objectives of this study were to develop a population pharmacokinetics model (POPPK) for gentamicin, designed for patients undergoing dialysis, and to investigate the best dosing scheme for different MIC clinical breakpoints using Monte Carlo simulations.
Methods
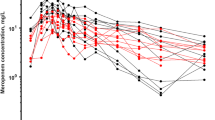
In this monocentric prospective interventional open clinical study, 23 patients (141 gentamicin samples) were included. The covariates investigated were weight, creatinine, dialysis (yes/no), dialysis flow and dialysis duration. The POPPK model was developed in Pmetrics and 1000 time-concentration profiles were simulated for 9 doses between 2 and 10 mg/kg/day, with an inter-dose period of 24, 48 or 96 h to predict the probability of having both a serum peak > 8*MIC and a trough < 1 mg/L for MIC values between 0.25 and 4 mg/L.
Results
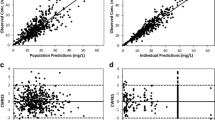
A two-compartment model including the dialysis on the elimination constant and bodyweight on the volume of distribution best described the data. A 30-min gentamicin infusion of 2 mg/kg/day (for MIC = 1 mg/L) or 8 mg/kg/day (for MIC = 4 mg/L) just before dialysis eliminated by two dialysis sessions before the next administration (dose interval of at least 96 h) led to a peak > 8*MIC for > 90% of the simulations and a trough concentration < 1 mg/L at 96 h for 92% and 34% respectively.
Conclusion
The gentamicin dose generally used to treat infections in dialysis patients is insufficient and might be increased to 3–8 mg/kg/day just before dialysis, taking into account the type of infection.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Becker B, Cooper MA (2013) Aminoglycoside antibiotics in the 21st century. ACS Chem Biol 8:105–115. https://doi.org/10.1021/cb3005116
Agence française de sécurité sanitaire des produits de santé (2012) Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect 42:301–308. https://doi.org/10.1016/j.medmal.2011.07.007
Wargo KA, Edwards JD (2014) Aminoglycoside-induced nephrotoxicity. J Pharm Pract 27:573–577. https://doi.org/10.1177/0897190014546836
Jiang M, Karasawa T, Steyger PS (2017) Aminoglycoside-induced cochleotoxicity: a review. Front Cell Neurosci 11. https://doi.org/10.3389/fncel.2017.00308
Moore RD, Lietman PS, Smith CR (1987) Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99
Berman SJ, Johnson EW, Nakatsu C, Alkan M, Chen R, LeDuc J (2004) Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clin Infect Dis 39:1747–1753. https://doi.org/10.1086/424516
Fresenius Kabi USA, FDA (2013) Product information: gentamicin intramuscular injection solution, intravenous injection solution, gentamicin intramuscular injection solution, intravenous injection solution. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/062366s033lbl.pdf. Accessed 24 Feb 2019
Wilson TW, Mahon WA, Inaba T, Johnson GE, Kadar D (1973) Elimination of tritiated gentamicin in normal human subjects and in patients with severely impaired renal function. Clin Pharmacol Ther 14:815–822
Vozeh S, Spring P, Wenk M, Follath F (1979) Changes in the apparent half life of gentamicin and tobramycin without detectable changes in creatinine clearance. Br J Clin Pharmacol 7:629–631. https://doi.org/10.1111/j.1365-2125.1979.tb04656.x
Rein - adaptation posologique. In: SiteGPR. http://sitegpr.com/fr/rein/recherche-par-medicaments/. Accessed 24 Aug 2018
ANSM (2011) Mise au point sur le bon usage des aminosides administres par voie injectable. In: ANSM. https://ansm.sante.fr/var/ansm_site/storage/original/application/3e0d2264e2921c8465d9ad6464e12660.pdf. Accessed 30 Aug 2018
Hodiamont CJ, Janssen JM, de Jong MD et al (2017) Therapeutic drug monitoring of gentamicin peak concentrations in critically ill patients. Ther Drug Monit 39:522–530. https://doi.org/10.1097/FTD.0000000000000432
Fuchs A, Guidi M, Giannoni E, Werner D, Buclin T, Widmer N, Csajka C (2014) Population pharmacokinetic study of gentamicin in a large cohort of premature and term neonates. Br J Clin Pharmacol 78:1090–1101. https://doi.org/10.1111/bcp.12444
Venisse N, Dupuis A, Badin J, Robert R, Pinsard M, Veinstein A (2015) Efficacy and safety of high-dose gentamicin re-dosing in ICU patients receiving haemodialysis. J Antimicrob Chemother 70:308–310. https://doi.org/10.1093/jac/dku369
Veinstein A, Venisse N, Badin J, Pinsard M, Robert R, Dupuis A (2013) Gentamicin in hemodialyzed critical care patients: early dialysis after administration of a high dose should be considered. Antimicrob Agents Chemother 57:977–982. https://doi.org/10.1128/AAC.01762-12
O’Shea S, Duffull S, Johnson DW (2009) Editorial: aminoglycosides in hemodialysis patients: is the current practice of post dialysis dosing appropriate? Semin Dial 22:225–230. https://doi.org/10.1111/j.1525-139X.2008.00554.x
Teigen MMB, Duffull S, Dang L, Johnson DW (2006) Dosing of gentamicin in patients with end-stage renal disease receiving hemodialysis. J Clin Pharmacol 46:1259–1267. https://doi.org/10.1177/0091270006292987
Dang L, Duffull S (2006) Development of a semimechanistic model to describe the pharmacokinetics of gentamicin in patients receiving hemodialysis. J Clin Pharmacol 46:662–673. https://doi.org/10.1177/0091270006286902
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW (2012) Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. https://doi.org/10.1097/FTD.0b013e31825c4ba6
Goutelle S, Bourguignon L, Maire PH, van Guilder M, Conte JE Jr, Jelliffe RW (2009) Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob Agents Chemother 53:2974–2981. https://doi.org/10.1128/AAC.01520-08
EUCAST: Clinical breakpoints and dosing of antibiotics. http://www.eucast.org/clinical_breakpoints/. Accessed 16 Oct 2019
Medellín-Garibay SE, Rueda-Naharro A, Peña-Cabia S, García B, Romano-Moreno S, Barcia E (2015) Population pharmacokinetics of gentamicin and dosing optimization for infants. Antimicrob Agents Chemother 59:482–489. https://doi.org/10.1128/AAC.03464-14
Rosario MC, Thomson AH, Jodrell DI et al (1998) Population pharmacokinetics of gentamicin in patients with cancer. Br J Clin Pharmacol 46:229–236. https://doi.org/10.1046/j.1365-2125.1998.00779.x
Zhuang L, He Y, Xia H, Liu Y, Sy SK, Derendorf H (2016) Gentamicin dosing strategy in patients with end-stage renal disease receiving haemodialysis: evaluation using a semi-mechanistic pharmacokinetic/pharmacodynamic model. J Antimicrob Chemother 71:1012–1021. https://doi.org/10.1093/jac/dkv428
Jelliffe R (2016) Challenges in individualizing drug dosage for intensive care unit patients: is augmented renal clearance what we really want to know? Some suggested management approaches and clinical software tools. Clin Pharmacokinet 55:897–905. https://doi.org/10.1007/s40262-016-0369-4
Proost JH (2017) Comment on: “Challenges in individualizing drug dosage for intensive care unit patients: is augmented renal clearance what we really want to know? Some suggested management approaches and clinical software tools.”. Clin Pharmacokinet 56:311–312. https://doi.org/10.1007/s40262-016-0480-6
Dahlgren JG, Anderson ET, Hewitt WL (1975) Gentamicin blood levels: a guide to nephrotoxicity. Antimicrob Agents Chemother 8:58–62. https://doi.org/10.1128/AAC.8.1.58
Jao RL, Jackson GG (1964) Gentamicin sulfate, new antibiotic against gram-negative bacilli: laboratory, pharmacological, and clinical evaluation. JAMA 189. https://doi.org/10.1001/jama.1964.03070110019004
Acknowledgements
We thank Helene Roussel, Alexandre Garnier and Chloé Barny for their precious help in the study management. We thank Karen Poole for manuscript editing.
Funding
We thank the DRCI of Limoges University Hospital for funding this study.
Author information
Authors and Affiliations
Contributions
CM, FSM, ME, JPR, JA, VA and PM conceived or designed the study; BF, JBW, CM, FSM, ME, JPR, JA, VA and PM performed research; and BF and JBW analysed data and wrote the paper.
Corresponding author
Ethics declarations
The ethics committee of Limoges Hospital approved the protocol (no. 201231120). All the patients included in the study gave their written informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Franck, B., Monchaud, C., Saint-Marcoux, F. et al. Population pharmacokinetics of gentamicin in haemodialysis patients: modelling, simulations and recommendations. Eur J Clin Pharmacol 76, 947–955 (2020). https://doi.org/10.1007/s00228-020-02867-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02867-3