Abstract
Purpose
The purpose of this study was to compare therapeutic vancomycin trough levels in obese adults when using an original nomogram (phase I) versus a modified nomogram (phase II).
Methods
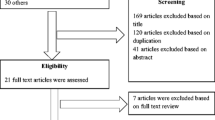
This study compared a vancomycin nomogram with a modified vancomycin nomogram for obese adults over 100 kg. The primary endpoint compared the percentage of sub-therapeutic, therapeutic, and supra-therapeutic vancomycin trough concentrations between the nomograms. Patients were included if they were at least 18 years of age, had a total body weight of 100–299 kg, and had an initial vancomycin trough level collected. Patients were excluded if they had end-stage renal disease or were on continuous renal replacement therapy.
Results
Therapeutic trough levels occurred in 85 out of 171 patients (50%) in phase I and 98 out of 149 patients (66%) in phase II. The incidence of both sub-therapeutic and supra-therapeutic troughs was less in phase II (p = 0.013). In the subgroup of adults aged 18 to 49 with a normalized creatinine clearance of greater than 90 mL/min, there was a trend in more therapeutic levels with the modified nomogram and less chance of sub-therapeutic levels (p = 0.088). In the subgroup of adults with a normalized creatinine clearance of 60–90 mL/min, there was significant improvement in therapeutic levels and a decrease in supra-therapeutic levels without increasing the percent of sub-therapeutic levels (p = 0.001).
Conclusion
The modified vancomycin nomogram at Cone Health showed significant improvement in therapeutic trough concentrations while reducing the rates of under and over dosing obese adults. The Cone Health–modified vancomycin nomogram could be a useful tool for initial dosing of vancomycin in the obese population.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to PHI, but are available from the corresponding author on reasonable request.
References
The Global BMI Mortality Collaboration (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388(10046):776–786
Falgas ME, Kompoti M (2006) Obesity and infection. Lancet Infect Dis 7:438–446
Centers for Disease Control and Prevention. Adult obesity facts. http://www.cdc.gov/obesity/data/adult.html. Updated August 13th, 2018. Accessed February 12th 2019
Janson B, Thursky K (2012) Dosing of antibiotics in obesity. Curr Opin Infect Dis 25(6):634–649
Tucker CE, Lockwood AM, Nguyen NH (2014) Antibiotic dosing in obesity: the search for optimum dosing strategies. Clinical Obesity 4:287–295
Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP (2009) Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 1:82–98
Bauer LA, Black DJ, Lill JS (1998) Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol 54:621–625
Blouin RA, Bauer LA, Miller DD, Record KE, Griffin WO (1982) Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother 21:575–580
Okamoto K, Seto TB, Davis J, Dement L, Bello EF (2013) Actual body weight dosing of vancomycin in obese patients. Hawaii Journal of Medicine and Public Health 72(9 Suppl 4):67
Reynolds DC, Waite LH, Alexander DP, DeRyke CA (2012) Performance of a vancomycin dosage regimen developed for obese patients. Am J Health Syst Pharm 69(11):944–950
Kubiak DW, Alquwaizani M, Sansonetti D, Barra ME, Calderwood MS (2015) An evaluation of systemic vancomycin dosing in obese patients. Open Forum Infectious Diseases 2(4):ofv176
Meng L, Mui E, Holubar MK, Deresinski SC (2017) Comprehensive guidance for antibiotic dosing in obese adults. Pharmacotherapy 37(11):1415–1431
Finch NA, Zasowski EJ, Murray KP, Mynatt RP, Zhao JJ, Yost R, Pogue JM, Rybak MJ (2017) The impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity: a quasi-experiment. Antimicrob Agents Chemother 61(12):e01293–e01217
Demirovic JA, Pai AB, Pai MP (2009) Estimation of creatinine clearance in morbidly obese patients. American Journal of Health System Pharmacy 66(7):642–648
Salazar DE, Corcoran GB (1988) Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med 84:1053–1060
Acknowledgments
The authors would like to thank Randy Absher PharmD, for statistical analysis of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approval was obtained from the Cone Health institutional review board. This study conformed with the Helsinki Declaration of 1964, as revised in 2013, concerning human and animal rights. Springer’s informed consent policy was followed. No consent was needed from patients as this was a retrospective chart review regarding patients that were already initiated on vancomycin. No identifying information is included in this article.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Batchelder, N., Lutheran, C.F. & Frens, J. Evaluation of a modified vancomycin nomogram for obese adults. Eur J Clin Pharmacol 76, 403–408 (2020). https://doi.org/10.1007/s00228-019-02811-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02811-0