Abstract
Purpose
The partial ineffectiveness and side effects of inflammatory bowel disease (IBD) current therapies drive basic research to look for new therapeutic target in order to develop new drug lead. Considering the pivotal role played by toll-like receptors (TLRs) in gut inflammation, we evaluate here the therapeutic effect of the synthetic glycolipid TLR4 antagonist FP7.
Methods
The anti-inflammatory effect of FP7, active as TLR4 antagonist, was evaluated on peripheral blood mononuclear cells (PBMCs) and lamina propria mononuclear cells (LPMCs) isolated from IBD patients, and in a mouse model of ulcerative colitis.
Results
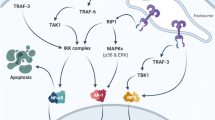
FP7 strongly reduced the inflammatory responses induced by lipopolysaccharide (LPS) in vitro, due to its capacity to compete with LPS for the binding of TLR4/MD-2 receptor complex thus inhibiting both the MyD88- and TRIF-dependent inflammatory pathways. Colitic mice treated with FP7 exhibit reduced colonic inflammation and decreased levels of pro-inflammatory cytokines.
Conclusions
This study suggests that TLR4 chemical modulation can be an effective therapeutic approach to IBD. The selectivity of FP7 on TLR4 makes this molecule a promising drug lead for new small molecules-based treatments.





Similar content being viewed by others
Abbreviations
- CD:
-
Crohn’s disease
- DAMPs:
-
Danger-associated molecular patterns
- DSS:
-
Dextran sulfate sodium
- IBD:
-
Inflammatory bowel disease
- IP:
-
Intraperitoneal
- LBP:
-
Lipopolysaccharide binding protein
- LPMCs:
-
Lamina propria mononuclear cells
- MyD88:
-
Myeloid differentiation primary response gene(88)
- N.S:
-
Non-stimulated
- PAMPs:
-
Pathogen-associated molecular patterns
- PBMCs:
-
Peripheral blood mononuclear cells
- PRRs:
-
Pattern recognition receptors
- TLR:
-
Toll-like receptor
- UC:
-
Ulcerative colitis
References
de Souza HSP, Fiocchi C (2015) Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13:13
Amiot A, Peyrin-Biroulet L (2014) Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Ther Adv Gastroenterol 8:66–82
Abraham C, Cho JH (2009) Inflammatory bowel disease. N Engl J Med 361:2066–2078
Kamada N, Seo S-U, Chen GY, Núñez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13:321
Zeng MY, Inohara N, Nuñez G (2016) Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol 10:18
Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM (2010) Intestinal macrophages and response to microbial encroachment. Mucosal Immunol 4:31
Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD (2005) Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 115:66–75
Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M, Sellers MT, Orenstein JM, Shimada T, Graham MF, Kubagawa H (2001) Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol 167:2651
Smythies LE, Shen R, Bimczok D, Novak L, Clements RH, Eckhoff DE, Bouchard P, George MD, Hu WK, Dandekar S, Smith PD (2010) Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J Biol Chem 285:19593–19604
Cario E (2010) Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis 16:1583–1597
Oostenbrug LE, Drenth JPH, de Jong DJ, Nolte IM, Oosterom E, van Dullemen HM, van der Linde K, te Meerman GJ, van der Steege G, Kleibeuker JH, Jansen PLM (2005) Association between toll-like receptor 4 and inflammatory bowel disease. Inflamm Bowel Dis 11:567–575
Cario E, Podolsky DK (2000) Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 68:7010
Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, Arditi M (2002) TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem 277:20431–20437
Cario E, Podolsky DK (2005) Intestinal epithelial TOLLerance versus inTOLLerance of commensals. Mol Immunol 42:887–893
Poltorak A, He X, Smirnova I, Liu M-Y, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085
Gioannini TL, Weiss JP (2007) Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res 39:249–260
Heumann D, Lauener R, Ryffel B (2003) The dual role of LBP and CD14 in response to Gram-negative bacteria or Gram-negative compounds. J Endotoxin Res 9:381–384
Miyake K (2003) Innate recognition of lipopolysaccharide by CD14 and toll-like receptor 4-MD-2: unique roles for MD-2. Int Immunopharmacol 3:119–128
Gay NJ, Symmons MF, Gangloff M, Bryant CE (2014) Assembly and localization of toll-like receptor signalling complexes. Nat Rev Immunol 14:546
Lorne E, Dupont H, Abraham E (2010) Toll-like receptors 2 and 4: initiators of non-septic inflammation in critical care medicine? Intensive Care Med 36:1826–1835
Erridge C (2010) Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol 87:989–999
Cighetti R, Ciaramelli C, Sestito SE, Zanoni I, Kubik Ł, Ardá-Freire A, Calabrese V, Granucci F, Jerala R, Martín-Santamaría S, Jiménez-Barbero J, Peri F (2014) Modulation of CD14 and TLR4·MD-2 activities by a synthetic lipid A mimetic. ChemBioChem. 15:250–258
Perrin-Cocon L, Aublin-Gex A, Sestito SE, Shirey KA, Patel MC, André P, Blanco JC, Vogel SN, Peri F, Lotteau V (2017) TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci Rep 7:40791–40791
Facchini FA, Zaffaroni L, Minotti A, Rapisarda S, Calabrese V, Forcella M, Fusi P, Airoldi C, Ciaramelli C, Billod J-M, Schromm AB, Braun H, Palmer C, Beyaert R, Lapenta F, Jerala R, Pirianov G, Martin-Santamaria S, Peri F (2018) Structure–activity relationship in monosaccharide-based toll-like receptor 4 (TLR4) antagonists. J Med Chem 61:2895–2909
Grasselli C, Ferrari D, Zalfa C, Soncini M, Mazzoccoli G, Facchini FA, Marongiu L, Granucci F, Copetti M, Vescovi AL, Peri F, De Filippis L (2018) Toll-like receptor 4 modulation influences human neural stem cell proliferation and differentiation. Cell Death Dis 9:280
Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, Tersigni R, Alessandroni L, Biancone L, Naccari GC, MacDonald TT, Pallone F (2005) Interleukin-21 enhances T-helper cell type I signaling and interferon-γ production in Crohn’s disease. Gastroenterology. 128:687–694
Franchi L, Monteleone I, Hao L-Y, Spahr MA, Zhao W, Liu X, Demock K, Kulkarni A, Lesch CA, Sanchez B, Carter L, Marafini I, Hu X, Mashadova O, Yuan M, Asara JM, Singh H, Lyssiotis CA, Monteleone G, Opipari AW, Glick GD (2017) Inhibiting oxidative phosphorylation in vivo restrains Th17 effector responses and ameliorates murine colitis. J Immunol 198:2735
Grimm MC, Pavli P, Van De Pol E, Doe WF (1995) Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa—implications for pathogenesis. Clin Exp Immunol 100:291–297
Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14:329
Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R (2008) TRAM couples endocytosis of toll-like receptor 4 to the induction of interferon-β. Nat Immunol 9:361
Tan Y, Zanoni I, Cullen TW, Goodman AL, Kagan JC (2015) Mechanisms of toll-like receptor 4 endocytosis reveal a common immune-evasion strategy used by pathogenic and commensal bacteria. Immunity. 43:909–922
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499
Zhao X-J, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK (2008) TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol 181:3049
Moore TC, Cody L, Kumm PM, Brown DM, Petro TM (2013) IRF3 helps control acute TMEV infection through IL-6 expression but contributes to acute hippocampus damage following TMEV infection. Virus Res 178:226–233
de Mattos BRR, Garcia MPG, Nogueira JB, Paiatto LN, Albuquerque CG, Souza CL, Fernandes LGR, Tamashiro WM, Simioni PU (2015) Inflammatory bowel disease: an overview of immune mechanisms and biological treatments. Mediat Inflamm 2015:493012–493012
Targan SR, Hanauer SB, Van Deventer SJH, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ (1997) A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Engl J Med 337:1029–1035
Prantera C (2008) What role do antibiotics have in the treatment of IBD? Na Clin Pract Gastroenterol Hepatol 5:670
Fukata M, Arditi M (2013) The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol 6:451
Liu Y, Zhang Z, Wang L, Li J, Dong L, Yue W, Chen J, Sun X, Zhong L, Sun D (2010) TLR4 monoclonal antibody blockade suppresses dextran-sulfate-sodium-induced colitis in mice. J Gastroenterol Hepatol 25:209–214
Ungaro R, Fukata M, Hsu D, Hernandez Y, Breglio K, Chen A, Xu R, Sotolongo J, Espana C, Zaias J, Elson G, Mayer L, Kosco-Vilbois M, Abreu MT (2009) A novel toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol-Gastrointest Liver Physiol 296:G1167–G1179
Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 338:120
Barnich N, Darfeuille-Michaud A (2007) Adherent-invasive Escherichia coli and Crohn’s disease. Curr Opin Gastroenterol 23:16–20
Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB (2007) Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2:119–129
Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Núñez G (2016) Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity. 44:647–658
Levin A, Shibolet O (2008) Toll-like receptors in inflammatory bowel disease-stepping into uncharted territory. World J Gastroenterol 14:5149–5153
Acknowledgments
The Italian Ministry for Foreign Affairs and International Cooperation (MAECI).
Author contribution statement
Ivan Monteleone, Francesco Peri, Giovanni Monteleone, and Fabio A. Facchini designed the study. Fabio A. Facchini performed all the experimental work presented with the help of Davide Di Fusco and Simona Barresi. In particular, in vivo experimentation and processing of patients’ biopsy and specimens were performed with Davide Di Fusco, while western blot analysis was performed with the help of Simona Barresi and Francesca Granucci. Andrea Luraghi and Alberto Minotti synthesized the molecule FP7.
Funding
Project H2020-MSC-ETN-642157 TOLLerant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Facchini, F.A., Di Fusco, D., Barresi, S. et al. Effect of chemical modulation of toll-like receptor 4 in an animal model of ulcerative colitis. Eur J Clin Pharmacol 76, 409–418 (2020). https://doi.org/10.1007/s00228-019-02799-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02799-7