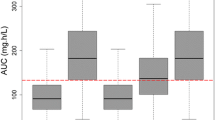
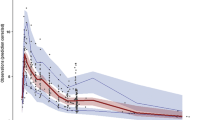
Abstract
Objectives
The study aims to assess the population pharmacokinetics of fluconazole and the adequacy of current dosages and breakpoints against Candida albicans and non-albicans spp. in liver transplant (LT) patients.
Patients and methods
Patients initiated i.v. fluconazole within 1 month from liver transplantation (LTx) for prevention or treatment of Candida spp. infections. Multiple assessments of trough and peak plasma concentrations of fluconazole were undertaken in each patient by means of therapeutic drug monitoring. Monte Carlo simulations were performed to define the probability of target attainment (PTA) with a loading dose (LD) of 400, 600, and 800 mg at day 1, 7, 14, and 28 from LTx, followed by a maintenance dose (MD) of 100, 200, and 300 mg daily of the pharmacokinetic/pharmacodynamic target of AUC24h/MIC ratio ≥ 55.2.
Results
Nineteen patients were recruited. A two-compartment model with first-order intravenous input and first-order elimination was developed. Patient’s age and time elapsed from LTx were the covariates included in the final model. At an MIC of 2 mg/L, a LD of 600 mg was required for optimal PTAs between days 1 and 20 from LTx, while 400 mg was sufficient from days 21 on. A MD of 200 mg was required for patients aged 40–49 years old, while a dose of 100 mg was sufficient for patients aged ≥ 50 years.
Conclusions
Fluconazole dosages of 100–200 mg daily may ensure optimal PTA against C. albicans, C. parapsilosis, and C. tropicalis. Higher dosages are required against C. glabrata. Estimated creatinine clearance is not a reliable predictor of fluconazole clearance in LT patients.





Similar content being viewed by others
References
Saliba F, Delvart V, Ichai P, Kassis N, Botterel F, Mihaila L et al (2013) Fungal infections after liver transplantation: outcomes and risk factors revisited in the MELD era. Clin Transpl 27(4):E454–E461
Nagao M, Fujimoto Y, Yamamoto M, Matsumura Y, Kaido T, Takakura S, Uemoto S, Ichiyama S (2016) Epidemiology of invasive fungal infections after liver transplantation and the risk factors of late-onset invasive aspergillosis. J Infect Chemother 22(2):84–89
Hogen R, Dhanireddy KK (2017) Invasive fungal infections following liver transplantation. Curr Opin Organ Transplant 22(4):356–363
Andes DR, Safdar N, Baddley JW, Alexander B, Brumble L, Freifeld A, Hadley S, Herwaldt L, Kauffman C, Lyon GM, Morrison V, Patterson T, Perl T, Walker R, Hess T, Chiller T, Pappas PG, The TRANSNET Investigators (2016) The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the transplant-associated infection surveillance network (TRANSNET). Transpl Infect Dis 18(6):921–931
Bassetti M, Peghin M, Carnelutti A, Righi E, Merelli M, Ansaldi F, Trucchi C, Alicino C, Sartor A, Wauters J, Lagrou K, Tascini C, Menichetti F, Mesini A, de Rosa FG, Lagunes L, Rello J, Colombo AL, Vena A, Munoz P, Tumbarello M, Sganga G, Martin-Loeches I, Viscoli C (2017) Invasive Candida infections in liver transplant recipients: clinical features and risk factors for mortality. Transplant Direct 3(5):e156
Evans JD, Morris PJ, Knight SR (2014) Antifungal prophylaxis in liver transplantation: a systematic review and network meta-analysis. Am J Transplant 14(12):2765–2776
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD (2016) Clinical practice guideline for the Management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62(4):e1–e50
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group (2012) ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):19–37
Sabatelli F, Patel R, Mann PA, Mendrick CA, Norris CC, Hare R, Loebenberg D, Black TA, McNicholas PM (2006) In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother 50(6):2009–2015
Bellmann R (2007) Clinical pharmacokinetics of systemically administered antimycotics. Curr Clin Pharmacol 2(1):37–58
Fortun J, Muriel A, Martin-Davila P, Montejo M, Len O, Torre-Cisneros J et al (2016) Caspofungin versus fluconazole as prophylaxis of invasive fungal infection in high-risk liver transplantation recipients: a propensity score analysis. Liver Transpl 22(4):427–435
Giannella M, Husain S, Saliba F, Viale P (2018) Use of echinocandin prophylaxis in solid organ transplantation. J Antimicrob Chemother 73(suppl_1):i51–ii9
Scudeller L, Viscoli C, Menichetti F, del Bono V, Cristini F, Tascini C, Bassetti M, Viale P, ITALIC Group (2014) An Italian consensus for invasive candidiasis management (ITALIC). Infection 42(2):263–279
Viscoli C (2009) Antifungal prophylaxis and pre-emptive therapy. Drugs 69(Suppl 1):75–78
Pai MP, Turpin RS, Garey KW (2007) Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob Agents Chemother 51(1):35–39
Muhl E, Martens T, Iven H, Rob P, Bruch HP (2000) Influence of continuous veno-venous haemodiafiltration and continuous veno-venous haemofiltration on the pharmacokinetics of fluconazole. Eur J Clin Pharmacol 56(9–10):671–678
Rodriguez-Tudela JL, Almirante B, Rodriguez-Pardo D, Laguna F, Donnelly JP, Mouton JW, Pahissa A, Cuenca-Estrella M (2007) Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob Agents Chemother 51(10):3599–3604
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Flamant M, Haymann JP, Vidal-Petiot E, Letavernier E, Clerici C, Boffa JJ, Vrtovsnik F (2012) GFR estimation using the Cockcroft-Gault, MDRD study, and CKD-EPI equations in the elderly. Am J Kidney Dis 60(5):847–849
Inagaki K, Takagi J, Lor E, Okamoto MP, Gill MA (1992) Determination of fluconazole in human serum by solid-phase extraction and reversed-phase high-performance liquid chromatography. Ther Drug Monit 14(4):306–311
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW (2012) Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34(4):467–476
Masterton RG, Kuti JL, Turner PJ, Nicolau DP (2005) The OPTAMA programme: utilizing MYSTIC (2002) to predict critical pharmacodynamic target attainment against nosocomial pathogens in Europe. J Antimicrob Chemother 55(1):71–77
Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D (2010) Testing CSfAS. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat 13(6):180–195
Han S, Kim J, Yim H, Hur J, Song W, Lee J, Jeon S, Hong T, Woo H, Yim DS (2013) Population pharmacokinetic analysis of fluconazole to predict therapeutic outcome in burn patients with Candida infection. Antimicrob Agents Chemother 57(2):1006–1011
Aoyama T, Hirata K, Hirata R, Yamazaki H, Yamamoto Y, Hayashi H, Matsumoto Y (2012) Population pharmacokinetics of fluconazole after administration of fosfluconazole and fluconazole in critically ill patients. J Clin Pharm Ther 37(3):356–363
McLachlan AJ, Tett SE (1996) Pharmacokinetics of fluconazole in people with HIV infection: a population analysis. Br J Clin Pharmacol 41(4):291–298
Tett S, Moore S, Ray J (1995) Pharmacokinetics and bioavailability of fluconazole in two groups of males with human immunodeficiency virus (HIV) infection compared with those in a group of males without HIV infection. Antimicrob Agents Chemother 39(8):1835–1841
Debruyne D, Ryckelynck JP (1993) Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet 24(1):10–27
Blot SI, Pea F, Lipman J (2014) The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11
Henriksen JH, Kiszka-Kanowitz M, Bendtsen F (2002) Review article: volume expansion in patients with cirrhosis. Aliment Pharmacol Ther 16(Suppl 5):12–23
Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB (2004) Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl 10(2):301–309
Cantarovich M, Yoshida EM, Peltekian KM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Barkun J (2006) Poor prediction of the glomerular filtration rate using current formulas in de novo liver transplant patients. Transplantation 82(3):433–436
Gross AS, McLachlan AJ, Minns I, Beal JB, Tett SE (2001) Simultaneous administration of a cocktail of markers to measure renal drug elimination pathways: absence of a pharmacokinetic interaction between fluconazole and sinistrin, p-aminohippuric acid and pindolol. Br J Clin Pharmacol 51(6):547–555
Putt TL, Duffull SB, Schollum JB, Walker RJ (2014) GFR may not accurately predict aspects of proximal tubule drug handling. Eur J Clin Pharmacol 70(10):1221–1226
Naesens M, Kuypers DR, Sarwal M (2009) Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4(2):481–508
Andes D (2003) In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob Agents Chemother 47(4):1179–1186
Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW (2014) Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69(5):1162–1176
Lopez-Cortes LE, Almirante B, Cuenca-Estrella M, Garnacho-Montero J, Padilla B, Puig-Asensio M et al (2016) Empirical and targeted therapy of candidemia with fluconazole versus echinocandins: a propensity score-derived analysis of a population-based, multicentre prospective cohort. Clin Microbiol Infect 22(8):733–e1–8
Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ, Anidulafungin Study Group (2007) Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 356(24):2472–2482
Eschenauer GA, Carver PL, Lin SW, Klinker KP, Chen YC, Potoski BA, Shields RK, Clancy CJ, Nguyen MH, Lam SW (2013) Fluconazole versus an echinocandin for Candida glabrata fungaemia: a retrospective cohort study. J Antimicrob Chemother 68(4):922–926
Pea F, Righi E, Cojutti P, Carnelutti A, Baccarani U, Soardo G, Bassetti M (2014) Intra-abdominal penetration and pharmacodynamic exposure to fluconazole in three liver transplant patients with deep-seated candidiasis. J Antimicrob Chemother 69(9):2585–2586
Kyriakidis I, Tragiannidis A, Munchen S, Groll AH (2017) Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 16(2):149–165
Wang JL, Chang CH, Young-Xu Y, Chan KA (2010) Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother 54(6):2409–2419
Bronstein JA, Gros P, Hernandez E, Larroque P, Molinie C (1997) Fatal acute hepatic necrosis due to dose-dependent fluconazole hepatotoxicity. Clin Infect Dis 25(5):1266–1267
Wells C, Lever AM (1992) Dose-dependent fluconazole hepatotoxicity proven on biopsy and rechallenge. J Inf Secur 24(1):111–112
Acknowledgments
W. H. holds or has recently held research grants with F2G, AiCuris, Astellas Pharma, Spero Therapeutics, Matinas Biosciences, Antabio, Amplyx, Allecra, Auspherix, and Pfizer, and he holds awards from the National Institutes of Health, Medical Research Council, National Institute of Health Research, FDA, and the European Commission (FP7 and IMI). W. H. has received personal fees in his capacity as a consultant for F2G, Amplyx, Ausperix, Spero Therapeutics, Medicines Company, Gilead, and Basilea, and he is an Ordinary Council Member for the British Society of Antimicrobial Chemotherapy. M. B. has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, AstraZeneca, Bayer, Basilea, Gilead, Menarini, MSD, Pfizer, The Medicines Company, Tetraphase, and Vifor. F. P. has received speaker honoraria from and attended advisory boards for Basilea Pharmaceutics, Gileads, MSD, and Pfizer.
Funding
This study was conducted as part of our routine work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Key points
• Fluconazole still remains the drug of choice for antifungal prophylaxis or treatment against C. albicans, C. parapsilosis, and C. tropicalis in clinically stable liver transplant (LT) patients.
• Estimated creatinine clearance does not have an impact on fluconazole clearance in the LT population.
• In LT patients in the first month from liver transplantation, the attainment of a pharmacodynamic target for efficacy is possible with dosages as low as 100–200 mg daily.
Electronic supplementary material
ESM 1
(DOCX 246 kb)
Rights and permissions
About this article
Cite this article
Cojutti, P.G., Lugano, M., Righi, E. et al. Population pharmacokinetics of fluconazole in liver transplantation: implications for target attainment for infections with Candida albicans and non-albicans spp.. Eur J Clin Pharmacol 74, 1449–1459 (2018). https://doi.org/10.1007/s00228-018-2526-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2526-1