Abstract
Purpose
Physicians often face difficulties in choosing appropriate medications for multimorbid older people. The FORTA (Fit for the Aged) classification (A: absolutely, B: beneficial, C: careful, D: don’t) was proposed as a clinical tool for improving the quality of drug treatment in the aged. As an implicit tool, FORTA has been shown to aid medication optimization and improve clinical end points in the VALFORTA trial. In this prospective randomized controlled study, 207 older hospitalized patients received standard geriatric treatment and 202 patients received FORTA-guided treatment.
Methods
Here, changes of drug prescriptions at the anatomical-therapeutic-chemical system (ATC) level were evaluated separately for important diagnoses in descriptive analyses; over- and under-treatment rates were compared between groups.
Results
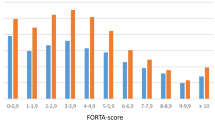
At the individual drug/drug class level related to all important diagnoses, the application of FORTA significantly improved under-treatments for 12 drugs/drug classes (e.g., ACE inhibitors to treat arterial hypertension) and over-treatments for 7 drugs/drug classes (e.g., proton pump inhibitors to treat gastroesophageal reflux disease).
Conclusions
FORTA representing the first combined positive/negative labeling approach at the individual drug level aids the optimization of drug treatment in older people as detected for drugs/drug classes at the ATC level in important indications. FORTA is effective in addressing over- and under-treatments even if analyzed for smaller subgroups of VALFORTA.
Similar content being viewed by others
References
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B (2012) Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380(9836):37–43. https://doi.org/10.1016/S0140-6736(12)60240-2
Formiga F, Ferrer A, Sanz H, Marengoni A, Alburquerque J, Pujol R, Octabaix study members (2013) Patterns of comobidity and multimorbidity in the oldest old: the Octabaix study. Eur J Intern Med 24(1):40–44. https://doi.org/10.1016/j.ejim.2012.11.003
Maher R, Hanlon J, Hajjar ER (2014) Clinical consequences of polypharmacy in elderly. Exp Opin Drug Saf 13(1):57–65. https://doi.org/10.1517/14740338.2013.827660
Johansson T, Abuzahra ME, Keller S, Mann E, Faller B, Sommerauer C, Höck J, Löffler C, Köchling A, Schuler J, Flamm M, Sönnichsen A (2016) Impact of strategies to reduce polypharmacy on clinically relevant endpoints—a systematic review and meta-analysis. Br J Clin Pharmacol 82(2):532–548. https://doi.org/10.1111/bcp.12959
Bokhof B, Junius-Walker U (2016) Reducing polypharmacy from the perspectives of general practitioners and older patients: a synthesis of qualitative studies. Drugs Aging 33(4):249–266. https://doi.org/10.1007/s40266-016-0354-5
Osburn R, Moulds D, Squires D, Doty MM, Anderson C (2014) International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff (Millwood) 33(12):2247–2255. https://doi.org/10.1377/hlthaff.2014.0947
Onder G, van der Cammen TJ, Petrovic M, Somers A, Rajkumar C (2013) Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing 42(3):284–291. https://doi.org/10.1093/ageing/aft038
Wehling M (2013) Drug therapy for the elderly. Springer Publisher, Vienna. ISBN 978-3-7091-0911-3
Katzung BG (2015) Basic & clinical pharmacology. 13th ed. Lange Medical Books/McGraw Hill, New York. ISBN 978-00718-2505-4
Van Spall HG, Toren A, Kiss A, Fowler RA (2007) Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 297(11):1233–1240. https://doi.org/10.1001/jama.297.11.1233
Shenoy P, Harugeri A (2015) Elderly patients’ participation in clinical trials. Perspect Clin Res 6(4):184–189. https://doi.org/10.4103/2229-3485.167099
Denson AC, Mahipal A (2014) Participation of the elderly population in clinical trials: barriers and solutions. Cancer Control 21(3):209–214. https://doi.org/10.1177/107327481402100305
American Geriatrics Society (2012) Beers Criteria Update Expert Panel (2012) American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 60:616–631
Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D (2008) STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 46(02):72–83. https://doi.org/10.5414/CPP46072
Wehling M (2008) Drug therapy in the elderly: too much or too little, what to do? A new assessment system: fit for the aged FORTA. Dtsch med Wochenschr 133:2289–2291 [article in German]
Wehling M (2009) Multimorbidity and polypharmacy: how to reduce the harmful drug load and yet add needed drugs in the elderly? Proposal of a new drug classification: fit for the aged. J Am Geriatr Soc 57(3):560–561. https://doi.org/10.1111/j.1532-5415.2009.02131.x
Wehling M (2016) Older people, a plethora of drugs, and drug list approaches: useful, efficacious, or a waste of time? J Am Med Dir Assoc 17(12):1073–1075. https://doi.org/10.1016/j.jamda.2016.08.023
Kuhn-Thiel AM, Weiss C, Wehling M (2014) The FORTA authors/expert panel members. Consensus validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging 31(2):131–140. https://doi.org/10.1007/s40266-013-0146-0
Pazan F, Weiss C, Wehling M (2016) The FORTA (Fit fOR The Aged) list 2015: update of a validated clinical tool for improved pharmacotherapy in the elderly. Drugs Aging 33(6):447–449. https://doi.org/10.1007/s40266-016-0371-4
Wehling M, Burkhardt H, Kuhn-Thiel AM et al (2016) VALFORTA—a randomized trial to validate the FORTA (“Fit fOR The Aged”) classification. Age Ageing 45(2):262–267. https://doi.org/10.1093/ageing/afv200
WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health, ATC/DDD index 2016. http://www.whocc.no/atc_ddd_index/. Accessed 19 July 2017
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49(12):1373–1379. https://doi.org/10.1016/S0895-4356(96)00236-3
Long JS (1997) Regression models for categorical and limited dependent variables. Sage Publications, Thousand Oaks
German Institute of Medical Documentation and Information, 2016 Official version of the German Anatomical Therapeutic Chemical (ATC) Classification with defined daily doses (DDD). http://www.dimdi.de/dynamic/de/klassi/downloadcenter/atcddd/. Accessed 19 July 2017
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44(2):213–218. https://doi.org/10.1093/ageing/afu145
Gallagher PF, O’Connor MN, O’Mahony D (2011) Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 89(6):845–854. https://doi.org/10.1038/clpt.2011.44
Pasina L, Djade CD, Tettamanti M, Franchi C, Salerno F, Corrao S, Marengoni A, Marcucci M, Mannucci PM, Nobili A, REPOSI Investigators (2014) Prevalence of potentially inappropriate medications and risk of adverse clinical outcome in a cohort of hospitalized elderly patients: results from the REPOSI Study. J Clin Pharm Ther 39(5):511–515. https://doi.org/10.1111/jcpt.12178
Gallagher PF, O’Mahony D (2008) STOPP (Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 37(6):673–679. https://doi.org/10.1093/ageing/afn197
O’Connor MN, O’Sullivan D, Gallagher PF, Eustace J, Byrne S, O’Mahony D (2016) Prevention of hospital-acquired adverse drug reactions in older people using screening tool of older persons' prescriptions and screening tool to alert to right treatment criteria: a cluster randomized controlled trial. J Am Geriatr Soc 64(8):1558–1566. https://doi.org/10.1111/jgs.14312
Wauters M, Elseviers M, Vaes B, Degryse J, Dalleur O, Vander Stichele R, Christiaens T, Azermai M (2016) Too many, too few, or too unsafe? Impact of inappropriate prescribing on mortality, and hospitalization in a cohort of community-dwelling oldest old. Br J Clin Pharmacol 82(5):1382–1392. https://doi.org/10.1111/bcp.13055
Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, HYVET Study Group (2008) Treatment of hypertension in patients 80 years of age or older. N Engl J Med 358(18):1887–1898. https://doi.org/10.1056/NEJMoa0801369
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365(11):981–992. https://doi.org/10.1056/NEJMoa1107039
Funding
The study was funded by the DFG-German Research Foundation (WE 1184/15-1 to M.W. and H.B., FR2997/2-1 to H.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethic committees at the Medical Faculty Mannheim, Heidelberg University, and at the University of Witten-Herdecke.
Conflicts of interest
M.W. was employed by AstraZeneca R&D, Mölndal, as director of discovery medicine (=translational medicine) from 2003 to 2006, while on sabbatical leave from his professorship at the University of Heidelberg. Since returning to this position in January 2007, he has received lecturing and consulting fees from Sanofi-Aventis, Bayer, Boehringer-Ingelheim, Novartis, Takeda, Roche, Pfizer, Bristol-Myers, Daichii-Sankyo, Lilly, Otsuka, Novo-Nordisk, Shire, and LEO Pharma. H.F. received lecturing and consulting fees from Amgen. F.P., H.B., C.T., A.K.T., and C.W. declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pazan, F., Burkhardt, H., Frohnhofen, H. et al. Changes in prescription patterns in older hospitalized patients: the impact of FORTA on disease-related over- and under-treatments. Eur J Clin Pharmacol 74, 339–347 (2018). https://doi.org/10.1007/s00228-017-2383-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2383-3