Abstract
Purpose
In 2014, FDA released a warning for prescription of doripenem for ventilator-associated bacterial pneumonia due to unsatisfactory clinical cure rates. The present study explores if the observed lack of efficacy might be explained by insufficient target site pharmacokinetics in intensive care patients after two different infusion schemes.
Methods
Plasma and bronchoalveolar lavage sampling was performed in 16 intubated patients with pneumonia receiving doripenem either as 1-h or as 4-h infusion. Doripenem concentrations were measured at steady state in plasma over 8 h, bronchoalvoelar lavage was performed in each patient once either after 0 h, 2 h, 4 h or 6 h.
Results
In plasma, mean values of Cmax, Tmax and AUC0–8 were 16.87 mg/L, 0.69 h and 52.98 mg/L×h after 1 h of infusion, and 12.94 mg/L, 3.21 h and 70.64 mg/L×h after 4 h of infusion, respectively. While the later tmax in plasma was with delay mirrored in the lung, for ELF, much lower concentrations were observed (Cmax, Tmax and AUC0–8 after 1-h infusion of 4.6 mg/L, 2 h and 15.3 mg/L×h and after 4-h infusion 6.9 mg/L, 4 h and 14.8 mg/L×h).
Conclusion
The difference in plasma pharmacokinetics after 1-h and 4-h infusion reflects in the concentration versus time profile in the lung, but concentration at the target site was not only considerably lower but also subject to high inter-individual variability. We hypothesise that insufficient concentrations at the target site might have contributed to the previously described lack of clinical efficacy and confirmed the demand for assessment of target site pharmacokinetics in larger patient collectives.
Similar content being viewed by others
Introduction
Sometimes, important lessons can be learned from failures. Doripenem is a carbapenem antibiotic with broad spectrum activity against Gram-positive and Gram-negative bacteria initially approved for the treatment of complicated intra-abdominal and urinary tract infections, as well as in Europe for the therapy of nosocomial including ventilator-associated pneumonia (VAP). In 2012, a large clinical trial comparing 7 days of treatment with 1 g of doripenem as a 4-h infusion every 8 h and 10 days of treatment with 1 g of imipenem/cilastatin as a 1-h infusion every 8 h showed increased risk of death and lower clinical cure rates when using doripenem compared with imipenem in patients with VAP [1]. Due to these results the FDA included a warning about the use of doripenem in patients with VAP in the drug label in 2014 [2]. In the same year, the marketing authorisation holder withdrew the European marketing authorisation of doripenem for all indications due to commercial reasons [3].
Antimicrobial efficacy depends on the concentrations of an antibiotic at the infection site, which may differ substantially from plasma concentrations [4,5,6]. Knowledge on plasma pharmacokinetics alone is therefore often not sufficient to allow for the appropriate estimation of bacterial killing in the targeted compartment [4, 7]. This single-centre descriptive pharmacokinetic study investigated doripenem concentrations in plasma and—representing the target site—in the epithelial lining fluid (ELF) of the lung with the aim to determine whether insufficient tissue concentrations at the target site might explain the observed lack of efficacy. Furthermore, two different dosing schemata of doripenem, i.e. standard infusion over 1 h versus extended infusion over 4 h were examined for the initially approved dosing regimen of 500 mg t.i.d to explore if longer infusion times lead to better serum and potentially also to target site target achievement.
Methods
Study subjects and treatment groups
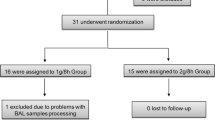
Sixteen intubated intensive care patients who received doripenem for treatment of pneumonia were included in this study. Patients were split into two groups of which one received doripenem 500 mg over 1 h, the other one over 4 h. Inclusion criteria were age above 18 years, the administration of doripenem for therapeutical or prophylactic reasons for nosocomial or community-acquired pneumonia, as well as mechanical ventilation via endotracheal tube. Main exclusion criteria were a known allergy or hypersensitivity against the study drug, hemofiltration or haemodialysis or a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen of less than 100 in combination with a positive end expiratory pressure of 20 cm of water or higher.
Plasma sampling
Intensive plasma sampling over 8 h was performed for determination of antibiotic concentrations at steady state after (mean) 8.3 doses (range 3–24 doses prior to the study dose) in the 1-h infusion cohort and after 5.0 doses in the 4-h infusion cohort (range 3–9 doses).
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed once in each patient at one of the time points 0, 2, 4 or 6 h after start of infusion by using the pre-existing endotracheal tube for administration of three 20 mL aliquots of saline and subsequently collecting fluid after each aliquot. The fluid which was collected after the first aliquot was dismissed and not included into analysis. Concentrations in BAL samples were corrected using the urea method (volume of ELF (mL) = [total amount of urea in lavage fluid retrieved (mg)] / [concentration of urea in plasma (mg/mL)]) [8] in order to calculate concentrations in ELF.
Analysis
The concentration of doripenem in plasma and ELF was determined by HPLC using a Dionex “UltiMate 3000” system (Dionex Corp., Sunnyvale, CA) with UV detection at 298 nm, chromatographic separation was carried out at 45 °C on a Hypersil BDS C18 column (5 μm, 250 × 4.6 mm I.D., Thermo Fisher Scientific, Inc., Waltham, MA), preceded by a Hypersil BDS C18 precolumn (5 μm, 10 × 4.6 mm I.D.). The mobile phase consisted of a continuous gradient mixed from ion-pair buffer, pH 3.0 (50 mM potassium phosphate with phosphoric acid and 5 mM heptanesulfonic acid (mobile phase A) and methanol (mobile phase B)). Calibration of the chromatogram was accomplished using the external standard method. Linear calibration curves were calculated from the peak areas of doripenem compared with the external standard by spiking drug-free human plasma and ELF with standard solutions of doripenem to obtain a concentration range of 0.01 to 10 μg/ml (average correlation coefficients > 0.99). The limit of quantification (LOQ) for doripenem in plasma and ultrafiltrate was 0.01 μg/ml. Coefficients of accuracy and precision for this compound were < 8.7%.
Pharmacokinetic calculations
One ELF concentration of doripenem per patient and in total, two concentrations per time point per cohort were finally obtained, and mean values at the different time points were calculated. Pharmacokinetic analysis for determination of Cmax, Tmax, and AUC was performed using Kinetica version 3.0, InnaPhase Corporation. Since doripenem has negligible protein binding, below 10% of total plasma concentrations were used for calculations [9].
Statistical calculation
Statistical calculation was performed using a commercially available programme IBM SPSS Statistics Version 20 (Armonk, NY, IBM Corp.). Correlation of creatinine clearance and AUC0–8 was calculated using Spearman correlation coefficient.
Pharmacodynamic considerations
Since doripenem—like all beta-lactams—belongs to the class of antibiotics which exhibit time-depending killing, time above the MIC50 and MIC90 (T>MIC) of two main Gram-negative pathogens causing pneumonia were calculated for the generated data. MIC values used for calculations were chosen as found in the trial conducted by Kollef et al. [1] showing MIC50 of 1 mg/L and MIC90 of 128 mg/L for Pseudomonas aeruginosa and a MIC50 value of 2 mg/L and MIC90 value of 64 mg/L for Acinetobacter baumanii. Time over the MIC was directly determined by measurement of strongly enlarged figures. The fact that in case of severe infection, a PK/PD target of four times of the MIC value (T >4xMIC) was previously suggested to achieve optimal therapeutic effect, was included into PK/PD considerations [10].
Results
Study subjects
Demographic data and a selection of laboratory parameters are shown in Table 1.
Pharmacokinetics
In plasma, mean values of Cmax, Tmax and AUC0–8 were 16.87 mg/L, 0.69 h and 52.98 mg/L×h after 1 h of infusion, and 12.94 mg/L, 3.21 h and 70.64 mg/L×h after 4 h of infusion, respectively. In ELF, Cmax, Tmax and AUC0–8 after infusion over 1 h were 4.6 mg/L, 2 h and 15.3 mg/L×h and after infusion over 4 h 6.9 mg/L, 4 h and 14.8 mg/L×h, respectively. Pharmacokinetic data for plasma and ELF are shown in Table 2 and Fig. 1. A significant correlation at the 0.01 level was found between creatinine clearance and AUC0–8 in the present study as shown in Fig. 2.
Mean (±SEM) concentration vs. time profiles of doripenem in plasma and in ELF obtained by BAL at 0, 2, 4, and 6 h after the start of doripenem infusion. Open symbols describe pharmacokinetics in plasma, closed symbols pharmacokinetics in ELF after an infusion duration of 1 h (squares) and 4 (diamonds) hours, respectively
Pharmacodynamics
In plasma, the investigated dosing regimens achieved mean T>MIC50 values of 78 and 61% for the 1-h infusion and values of 100 and 98% for the 4-h infusion for P. aeruginosa and A. baumanii, respectively. However, employing the target of four times over the MIC T >4xMIC, the 1-h infusion led to 39 and 24% T >4xMIC50 for P. aeruginosa and A. baumanii, respectively, whereas the extended infusion scheme achieved values of 81 and 45% of T >4xMIC50. Neither infusion scheme reached at any time point of the MIC90 values of the two pathogens.
Investigating T>MIC50 in ELF, neither one of the infusion schemata could lead to doripenem concentrations above 1 mg/L (equivalent to MIC50 of P. aeruginosa) over the full dosing interval, though both dosing regimens could provoke concentrations above 1 mg/L at the 2-h as well as the 4-h time point.
Discussion
We set out to investigate if insufficient concentrations at the infection site in the lung can explain the previous described failure of doripenem when treating VAP and whether longer infusion times can help to optimize target attainment both in plasma and at the target site. Due to the limited number of patient included and high variability of determined PK parameters, especially in the lung, these questions could only partially be answered. On the other hand, we were able to show that the observed variability intrinsically might be a factor that might have resulted in treatment failure in a relevant proportion of patients and that modification of the dosing regimen also reflects in target site PK.
In order to allow for a hypothesis explaining the insufficient efficacy of doripenem observed by Kollef et al. based on plasma PK, the following assumptions have to be made. First, as the actual MIC values of the pathogens that caused the VAP in that study were not available, only MIC50 levels could be used for PK/PD considerations. Second, individual PK profiles are not published for the study by Kollef et al., thus we could only estimate plasma exposure in that study by doubling plasma concentrations at every time point in all patients of the present study after the identical duration of infusion (4 h) to compensate for differences in dosing. Third, the fact that blood concentrations over four times the MIC might be needed in case of severe infections for beta-lactam antibiotics have to be considered [11]. Taking all these assumptions into account—including measuring time over MIC in figures after doubling plasma concentrations—the prolonged infusion regimen would have resulted in mean T >4xMIC50 of 81 and 45% for P. aeruginosa and A. baumanii, respectively. For beta-lactams, values between 40 and 60% T>MIC have been associated with optimal killing in in vivo and in vitro PK/PD models by Craig et al., [12] therefore, for both strains time over MIC50 would be in or above the range of 40–60%,suggesting that good antimicrobial efficacy might be expected.
Still, also in patients suffering from VAP caused by P. aeruginosa, the clinical cure rate was numerically lower for patients with P. aeruginosa VAP in the doripenem arm compared to the imipenem-cilastatin arm (41.2 versus 60.0%). Therefore, one might speculate that the observed lack of efficacy might be due to insufficient target site penetration in the critically ill population rather than with subtherapeutic plasma levels. Although not enough data for thorough PK-PD calculations for the lung was generated in the present study, a difference between the ELF concentration-time profiles after the two investigated infusion schemes was observed. As shown in Fig. 1, differences in dosing regimens and thereby modified plasma PK indeed reflects in changes in target site pharmacokinetics in ELF. However, none of the two profiles seem to sufficiently cover concentrations above the thresholds of 4 or 8 mg/L (= 4× MIC50 of pathogens with MIC 1 mg/L or 2 mg/L) which might explain why potential benefits of a prolonged infusion scheme did not translate into improved clinical endpoints when treating VAP.
For reliable determination of the time above the MIC in ELF, more BAL measurements, either by including more patients or by repeated samples per patient, would be necessary to permit PK-PD calculations and to compensate for the high inter-individual variability, i.e. differences in concentrations up to the factor eight at a single-time-point. Different factors like organ dysfunction, septic shock, concomitant medication or capillary leakage have been attributed to this variability [4, 7, 13, 14]. However, more BAL time points per patient might be problematic from the ethical point of view and repeated BAL procedure in one patient might falsify data. Moreover, while including more patients in this study would allow for better target attainment analysis for the overall population it might not change the main outcome, i.e. that inter-individual variability has to be expected to be very high, and despite the fact that plasma concentrations are mirrored in ELF, target site concentrations in an individual patient currently cannot be predicted.
A significant correlation has been found between creatinine clearance and AUC0–8 in the present study as shown in Fig. 2. Thereby, differences in creatinine clearance (112.4 vs. 76.2 mL/min for 1 and 4-h infusion, respectively) might also have contributed to higher AUC0–8 values found for the 4-h infusion regimen. In contrast to plasma pharmacokinetics, which is highly impacted by creatinine clearance, factors that are more difficult to measure, e.g. local inflammation in the lung or atelectasis, might additionally impact the target site penetration [15, 16]. Intracellular concentrations have not been determined in the present study, because beta-lactam antibiotics are not active against intracellular pathogens and no accumulation in phagocytes has been described [17]. Comparison of our data with pharmacokinetics in plasma and ELF determined by Justo et al. [18] in healthy adults after doripenem 500 mg administration as extended infusion allows a cautious conclusion on the difference between healthy subjects and patients. Plasma concentrations were up to 1.8-fold higher in patients than in healthy subjects (8.79 and 4.89 mg/L at 4.5-h time point). Likewise, Cmax in ELF of 6.93 mg/L (4-h time point) in patients was higher than in healthy volunteers 1.67 mg/dL (4-5 h time point), but most importantly variability was lower.
Our study thereby highlights the importance of determining infection site pharmacokinetics but most importantly the need for further exploration of factors impacting target site pharmacokinetics in the respective patient category as e.g. the individual lung penetration of antibiotics in patients with severe pneumonia.
In summary, our data suggest the potential benefit of prolonged infusion in terms of PK/PD indices in plasma in the investigated population of severely ill patients. While it was shown that differences in the concentration-time profile in plasma did transfer to the lung, the small sample size limits the information value of BAL data. We can only hypothesize that insufficient infection site concentrations might have contributed to a previously observed lack of efficacy. Nevertheless the study confirms the demand for assessment of target site concentrations of antibiotics as early and throughout antimicrobial drug development in order to avoid therapeutic failure despite plasma PK/PD targets are achieved.
References
Kollef MH, Chastre J, Clavel M, Restrepo MI, Michiels B, Kaniga K, Cirillo I, Kimko H, Redman R (2012) A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care 16(6):R218–R218. https://doi.org/10.1186/cc11862
FDA (Last Updated 2016) FDA Drug Safety Communication: FDA approves label changes for antibacterial Doribax (doripenem) describing increased risk of death for ventilator patients with pneumonia. https://www.fda.gov/Drugs/DrugSafety/ucm387971.htm. Accessed at 28 July 2017
EMA (2014) Public statement on Doribax http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2014/10/WC500175788.pdf. Accessed 28 July 2017
Zeitlinger MA, Dehghanyar P, Mayer BX, Schenk BS, Neckel U, Heinz G, Georgopoulos A, Muller M, Joukhadar C (2003) Relevance of soft-tissue penetration by levofloxacin for target site bacterial killing in patients with sepsis. Antimicrob Agents Chemother 47(11):3548–3553
Joukhadar C, Derendorf H, Muller M (2001) Microdialysis. A novel tool for clinical studies of anti-infective agents. Eur J Clin Pharmacol 57(3):211–219
Bellmann R, Kuchling G, Dehghanyar P, Zeitlinger M, Minar E, Mayer BX, Muller M, Joukhadar C (2004) Tissue pharmacokinetics of levofloxacin in human soft tissue infections. Br J Clin Pharmacol 57(5):563–568. https://doi.org/10.1111/j.1365-2125.2004.02059.x
Joukhadar C, Frossard M, Mayer BX, Brunner M, Klein N, Siostrzonek P, Eichler HG, Muller M (2001) Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med 29(2):385–391
Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG (1986) Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol(Bethesda, Md : 1985) 60(2):532–538
EMA Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000891/WC500037148.pdf. Accessed 28 July 2017
Mouton JW, den Hollander JG (1994) Killing of Pseudomonas Aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 38(5):931–936
Jamal JA, Roberts DM, Udy AA, Mat-Nor MB, Mohamad-Nor FS, Wallis SC, Lipman J, Roberts JA (2015) Pharmacokinetics of piperacillin in critically ill patients receiving continuous venovenous haemofiltration: a randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents 46(1):39–44. https://doi.org/10.1016/j.ijantimicag.2015.02.014
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26(1):1–10 quiz 11-12
Baniasadi S, Farzanegan B, Alehashem M (2015) Important drug classes associated with potential drug-drug interactions in critically ill patients: highlights for cardiothoracic intensivists. Ann Intensive Care 5(1):44. https://doi.org/10.1186/s13613-015-0086-4
Sime FB, Roberts MS, Roberts JA (2015) Optimization of dosing regimens and dosing in special populations. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis 21(10):886–893. https://doi.org/10.1016/j.cmi.2015.05.002
Stein GE, Wells EM (2010) The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia and complicated skin and soft-tissue infections caused by methicillin-resistant Staphylococcus Aureus: vancomycin and linezolid. Curr Med Res Opin 26(3):571–588. https://doi.org/10.1185/03007990903512057
Hutschala D, Kinstner C, Skhirtladze K, Mayer-Helm BX, Zeitlinger M, Wisser W, Muller M, Tschernko E (2008) The impact of perioperative atelectasis on antibiotic penetration into lung tissue: an in vivo microdialysis study. Intensive Care Med 34(10):1827–1834. https://doi.org/10.1007/s00134-008-1122-8
Tulkens PM (1991) Intracellular distribution and activity of antibiotics. Eur J Clin Microbiol Infect Dis 10(2):100–106. https://doi.org/10.1007/BF01964420
Justo J, Gotfried MH, Deyo K, Fischer P, Danzinger LH, K.A. Rodvold (2011) Doripenem intrapulmonary pharmacokinetics in healthy adult subjects. ICAAC Poster A1–1748
Acknowledgements
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee, in accordance with Austrian drug law and Declaration of Helsinki (EudraCT 2011–000035-89).
Statement of informed consent
Informed consent was sought as soon as consciousness and clinical condition allowed it.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oesterreicher, Z., Minichmayr, I., Sauermann, R. et al. Pharmacokinetics of doripenem in plasma and epithelial lining fluid (ELF): comparison of two dosage regimens. Eur J Clin Pharmacol 73, 1609–1613 (2017). https://doi.org/10.1007/s00228-017-2327-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2327-y