Abstract
Purpose
It has been reported recently that immune reactions are involved in the pathogenesis of certain types of adverse drug reactions (ADRs). We aimed to determine the associations between infections and drug-induced interstitial lung disease (DILD), rhabdomyolysis, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), or drug-induced liver injury (DILI) using a spontaneous adverse drug event reporting database in Japan.
Methods
The reported cases were classified into three categories (anti-infectious drug group, concomitant infection group, and non-infection group) based on the presence of anti-infectious drugs (either as primary suspected drug or concomitant drug) and infectious disease. We assessed the association between four severe ADRs and the presence and seriousness of infection using logistic regression analysis.
Results
We identified 177,649 cases reported in the study period (2009–2013). Logistic regression analysis showed significant positive associations between infection status and onset of SJS/TEN or DILI (SJS/TEN: anti-infectious drug group: odds ratio (OR) 2.04, 95% CI [1.85–2.24], concomitant infection group: OR 2.44, 95% CI [2.21–2.69], DILI: anti-infectious drug group: OR 1.27, 95% CI [1.09–1.49], concomitant infection group: OR 1.25, 95% CI [1.04–1.49]), compared to the non-infection group. By contrast, there were negative or no associations between infection and DILD or rhabdomyolysis. A significantly positive association between infection and SJS/TEN seriousness (OR 1.48, 95% CI [1.10–1.98]) was observed.
Conclusions
This study suggested that infection plays an important role in the development of SJS/TEN and DILI. For the patients with infection and/ or anti-infectious drugs, careful monitoring for severe ADRs, especially SJS/TEN, might be needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, Japan’s Pharmaceuticals and Medical Device Agency (PMDA) established a spontaneous reporting system (SRS), the Japanese Adverse Drug Event Report (JADER) database, a unitized large-scale database used to assess the risk of adverse drug reactions (ADRs). Medical staff handling drug have mandatory reporting requirements governed by regulation. The principal use of spontaneous adverse reporting database is to detect serious, unknown, and rare events as post-marketing surveillance. ADRs are a major problem in drug therapy. ADRs are generally classified into two types: type A (augmented) and B (bizarre) [1]. Type A ADRs are relatively common and often predictable and based on an exaggerated response to the primary and/or secondary pharmacological action of the drug. In contrast, type B ADRs are uncommon and unpredictable. Among type B ADRs, rhabdomyolysis, drug-induced interstitial lung disease (DILD), Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), and drug-induced liver injury (DILI) are severe, potentially fatal, and rare disorders.
DILD, ranging from benign infiltrates to life-threatening acute respiratory distress syndrome, is known to be caused by various types of drugs, including antineoplastic, antimicrobial, and antirheumatic agents [2]. The pathogenesis is thought to be a drug-induced direct lung injury or an immune-mediated reaction [3]. Rhabdomyolysis is a severe skeletal muscle injury that is often accompanied by acute renal failure and is sometimes fatal. This disorder is caused by a variety of drugs, but the most typical drug class is of lipid-lowering drugs such as statins and fibrates [4, 5]. SJS/TEN is an acute drug-induced condition associated with severe blistering, skin peeling, and multi-organ damage. Although the main cause is drugs, this ADR can be caused by infections [6]. DILI encompasses a spectrum of clinical diseases ranging from mild biochemical abnormalities to acute liver failure. Many classical drugs, including acetaminophen, anti-tuberculosis agents, anesthetic drugs of the halothane family, and non-steroidal anti-inflammatory drugs (NSAIDs), are known to cause DILI [7]. Although some plausible pathogenesis of development of these ADRs have been indicated, the pathogenesis of these ADRs is not fully understood. It has been suggested that the pathogenesis of some idiosyncratic ADRs involve the immune system, specifically human leukocyte antigens (HLAs) and the T-cells [8]. In addition, several studies have reported that antiviral therapy caused ADRs that were mediated via the immune system [6, 9]. Moreover, several studies, including case reports, have reported that drug-drug interactions between antibiotic, antifungal, and antiviral drugs cause severe ADRs [10, 11]. Since immune-mediated reactions are involved in the pathogenesis of the ADRs, a possible potentiation of these ADRs by infections via the resulting activated immune system is postulated.
Practically, it is difficult to perform a large-scale clinical study to detect very rare ADRs. Hence, we assessed the associations between these ADRs (rhabdomyolysis, DILD, SJS/TEN, and DILI) and the presence of infection using the spontaneous adverse drug event reporting database in Japan. Moreover, we investigated the association between the presence of infection and seriousness of these ADRs
Materials and methods
Data source
We utilized the Japanese Adverse Drug Event Report database (JADER), which is managed by the Pharmaceuticals and Medical Devices Agency (PMDA). Dataset can be accessed directly here: http://www.info.pmda.go.jp/fukusayoudb/CsvDownload.jsp (it is available in Japanese only), and are recorded in four relational tables: “DEMO,” “DRUG,” “REAC,” and “HIST.” The tables contained the following information: (1) DEMO table: ID number, reported number of times, sex, age, reporting year, type of report, and reporter information; (2) DRUG table: ID number, reported number of times, drug number, participation of drug, generic name, brand name, administration route, drug starting date, drug finishing date, dose, dosage unit, times per day of administration, reason of using drug, treatment methods, and relapse or not; (3) REAC table: ID number, reported number of times, name of ADR, progress, and date of onset; and (4) HIST table: ID number, reported number of times, and primary disease information. “DEMO” table was then connected to the “REAC,” “DRUG,” and “HIST” tables using the ID number of each recorded case. We utilized an additional dataset to convert the text data of the disease name and drug indication into the Medical Dictionary for Regulatory Activities (MedDRA)-preferred term (PT) codes in the connected JADER dataset. The additional data were provided by the Japan Pharmaceutical Information Center (JAPIC), Inc., (Tokyo, Japan).
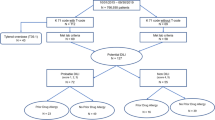
We used the November 2014 version of JADER and extracted the records of 177,659 cases of ADRs that were reported from 2009 through 2013. We classified the cases into three categories based on the presence of anti-infectious drugs (either as primary suspected drug or concomitant drug) and primary infectious disease as follows: (a) anti-infectious drug group: the case whose primary suspected drug was anti-infectious drug; (b) concomitant infection group: the cases whose primary suspected drug was not an anti-infectious drug but a concomitant drug and/or primary disease were anti-infection drugs and/or infectious diseases, respectively; and (c) non-infection group: the cases whose suspected drug and primary disease were neither anti-infectious drugs nor infectious diseases.
Target severe ADRs
The target severe ADRs were DILD, rhabdomyolysis, SJS/TEN, and DILI, and we defined these target ADRs based on the preferred terms (PTs) provided by the Medical Dictionary of Regulatory Activities, in which ADRs, medical history, and indications are coded (MedDRA, http://www.meddra.org). This dictionary is built as a five-level scale comprising 26 System Organ Classes (SOC) divided into High-Level Group Terms (HLGT), High-Level Terms (HLT), Preferred Terms (PT), and finally Lowest Level Terms (LLT).
We used MedDRA version 17.1. Selected PTs to define each target ADR are shown as follows: DILD (DILD: 10022611), rhabdomyolysis (rhabdomyolysis: 10039020), SJS/TEN (SJS: 10042033, TEN: 10044223), and oculomucocutaneous syndrome: 10030081, DILI (DILI: 10072268). We defined cases with at least one severe ADR as severe ADR cases.
Seriousness of target ADRs
We defined the cases whose clinical outcome was “death,” “unrecovered,” or “with sequela” as serious.
Anti-infectious drugs
Anti-infectious drugs were categorized based on the Japan Standard Commodity Classification (JSCC) SCC codes are composed of five or six digits. Therapeutic category numbers are decided based on three digits after the number “87.” The digit “6” after “87” indicates anti-infectious drugs. This classification system divides drugs into different groups according to their pharmacological actions, chemical structures, indications, or application sites. In some cases, drugs with the same indication are separately assigned into different categories according to their pharmacological actions or chemical structures. In other cases, a drug used for two or more indications is classified into more than one category. For example, amantadine has three indications in Japan (influenza A, Parkinsonism, and extrapyramidal reactions). Therefore, we re-classified drugs with two or more indications into one category after confirming the purpose of use through a manual search. The table, which corresponded to World Health Organization (WHO)-Anatomical Therapeutic Chemical (ATC) code, are shown as Appendix 1.
Infectious disease
Infectious diseases were defined using MedDRA terms. We adopted infection-related PTs under the SOC “infections and infestations” and test positive terms under HLGT “Microbiology and serology investigations” (e.g., MedDRA PT code: 10059421, bacterial test positive).
Potential confounding factors
The following variables were chosen as potential confounding factors: age, sex, reporter type, reporting type, reporting year, and the number of prescribed drugs. Age was categorized into three groups (age group 1: newborn, infant, and under the age of 20, age group 2: adults aged 20s to 60s, age group 3: elder, 70s to centenarians). Reporter types were categorized into four groups: physician, pharmacist, co-medical, consumer, and others (lawyer, etc.). Reporting type was categorized into four groups: clinical trial, spontaneous report, other, and unknown. In sensitivity analysis, we adjusted for class of drugs, which were classified based on the JSCC classification.
Statistical methods
All analyses were performed using STATA statistical software, version 14.2 (StataCorp, College Station, TX). For continuous variables, results were expressed as the mean ± standard deviation, and differences among groups were evaluated using the Kruskal-Wallis test. Categorical variables were represented as frequency counts, and intergroup comparisons were tested for significance using the χ 2 test. The associations between infection and the four target severe ADRs or seriousness of the target severe ADRs were evaluated by logistic regression analysis. Results are presented as the odds ratios (ORs) and 95% confidential intervals (95% CIs).
The class of concomitant drug was strongly related to that of primary suspected drug, and thus, some drug class might be preferentially administered with anti-infectious drug (e.g., antipyretics or antalgic drugs are frequently used in combination with anti-infectious drug for treatment of inflammatory conditions). Hence, we carried out sensitivity analyses on the concomitant infection group to minimize the potential impact of primary suspected drug types. We ruled out the anti-infectious drug group and adjusted for the drug types according to indications/pharmacological actions of primary suspected drugs based on JSCC classification. A P value < 0.05 was considered statistically significant.
Results
We identified 177,649 reported cases in the study period. Among them, 27,433 cases (15.4%) took anti-infectious drugs (anti-infectious drug group) and 30,253 cases (17.0%) were those whose primary suspected drug was not an anti-infectious drug but a concomitant drug and/or primary disease was an anti-infectious drug and/or infectious disease, respectively (concomitant infection group). The majority of reports (119,963 cases; 67.5%) neither took anti-infectious drugs nor suffered from an infectious disease (non-infection group).
We investigated the basic characteristics of cases that reported the target severe ADRs and classified each ADR group into three subgroups (Tables 1, 2, 3, and 4). Regarding DILD, significant differences between reports with DILD and three subgroups were found in sex (male and female), age (age 1, age 2, and age 3), report type (spontaneous report and clinical trial), and reporter (physician, pharmacist, co-medical). There were significant differences in the number of concomitant drugs taken in the concomitant infection subgroup and non-infection subgroup (P < 0.001). Regarding rhabdomyolysis, there were significant differences in sex (male and female), age (age 1, age 2, and age 3), report type (spontaneous report), and reporter (physician). Regarding SJS/TEN, there were significant differences in sex (male and female), age (age 1, age 2, and age 3), serious case, report type (spontaneous report, clinical trial, and other), and reporter (physician, pharmacist, co-medical, and consumer). Regarding DILI, there were significant differences in sex (male), age (age 2 and age 3), report type (spontaneous report and other), and reporter (physician, pharmacist, and consumer). Significant differences in the number of serious cases of SJS/TEN and DILI were found between the three subgroups (SJS/TEN: P < 0.001, DILI: P < 0.001).
To elucidate the association between infection and each severe ADR, we estimated the odds ratios (ORs) using logistic regression analysis (Table 5). The ORs of all variables in the models are indicated in Appendix 2. There was a significant negative association between the anti-infectious drug group and DILD compared to the non-infection group, although we did not find a significant association between the concomitant infection group and DILD (anti-infectious drug group: OR 0.51, 95% CI [0.47–0.56], concomitant infection group: OR 1.06, 95% CI [0.99–1.12]). There were significant negative associations between rhabdomyolysis and anti-infectious drugs or concomitant infections when compared to the non-infection group (anti-infectious drug group: OR 0.81, 95% CI [0.69–0.94], concomitant infection group: OR 0.77, 95% CI [0.67–0.90]). Positive associations between infection and both SJS/TEN and DILI were observed (SJS/TEN: anti-infective drug group: OR 2.04 95% CI [1.85–2.24], concomitant infection group: OR 2.44 95% CI [2.21–2.69]; DILI: anti-infective drug group: OR 1.27 95% CI [1.09–1.49], concomitant infection group: OR 1.25 95% CI [1.04–1.49]). Additionally, we showed the results on drug most frequently reported for the ADRs as Appendix 3.
We carried out a sensitivity analysis to minimize the potential impact of drug types that are preferentially used with anti-infective drugs. We adjusted the classification according to pharmacological actions of primary suspected drugs based on JSCC classification. Although rhabdomyolysis negatively associated with infection, there was a positive association between infection and DILD, SJS/TEN, or DILI (rhabdomyolysis: OR 0.79, 95% CI [0.68–0.92] P = 0.000, DILD: OR 1.13, 95% CI [1.05–1.20] P = 0.002, SJS/TEN: OR 2.56, 95% CI [2.31–2.83] P = 0.000, DILI: OR 1.21, 95% CI [1.01–1.45] P = 0.004).
Moreover, we assessed the association between the seriousness of ADR (clinical outcomes) and infection by each ADR cases. As shown in Table 6, there were significant associations between anti-infectious drugs or concomitant infection and seriousness of SJS/TEN (anti-infectious drug group: OR 1.61, 95% CI [1.21–2.15] P = 0.001, concomitant infection group: OR 1.48, 95% CI [1.10–1.98] P = 0.009). No associations between other three ADRs and infection were found.
Discussion
We analyzed the association between infection and four severe type B ADRs, DILD, rhabdomyolysis, SJS/TEN, and DILI, using the spontaneous adverse reporting database in Japan. Cases of DILD and rhabdomyolysis with concomitant anti-infectious drugs or infection had lower OR than those without infection, while cases of SJS/TEN and DILI with concomitant anti-infectious drugs or infection had significantly higher OR than those without infection. Moreover, we carried out a sensitivity analysis to minimize the impact of the type of the drug preferentially used as an anti-infectious drug. These associations remained significant in the sensitivity analysis. In addition, the data indicated that infection is associated with seriousness (serious clinical outcomes) of SJS/TEN. In particular, we observed that the association between infection and TEN, which has a more severe phenotype than SJS, was a significantly strong positive association only in the concomitant infectious group compared to the non-infection group (data not shown). Therefore, these findings suggested that onsets of SJS/TEN and DILI, but not DILD or rhabdomyolysis, could be potentiated by infection.
Few pharmacoepidemiological studies about the association between these ADRs and infection have examined. Regarding DILD, several case reports have reported interstitial lung disease in patients with prior history of mycobacterium tuberculosis infection [12] and after treatment for hepatitis C virus infection [13]. For rhabdomyolysis, Koubar et al. have reported that HIV-positive persons have high incidence of rhabdomyolysis [14]. It has been reported that SJS/TEN is caused by antibiotics or infection [15, 16]. Regarding DILI, it has been reported that anti-tuberculosis treatment for hepatitis B and C virus infection causes DILI [17, 18]. A Korean study has reported that anti-infective is one of most common suspected drugs of spontaneously reported hepatic ADRs [19].
The pathogenesis of these ADRs is still incompletely understood, although several mechanisms have been suggested. Our hypothesis is that the interaction between drugs and the immune response that was activated by infection plays an important role in the onset of these ADRs. The involvement of immune system-mediated reactions against drugs has been demonstrated as a mechanism of SJS/TEN and a certain type of DILI development [20, 21]. It is known that HLA molecules present intracellular or extracellular antigens to T-cell receptors, resulting in T-cell activation. Recently, a pharmacogenetic association of idiosyncratic ADRs has been found. There is increasing evidence that specific HLA alleles influence the risk of developing these ADRs, especially SJS/TEN and DILI [21,22,23,24,25]. Therefore, T-cell-mediated immune responses may play an important role in the pathogenesis of several immune-mediated ADRs. In line with this, infection may potentiate the expression of co-stimulatory molecules such as CD80 and CD86, which are known to be necessary for T-cell activation in addition to HLA molecules [26]. Thus, it is reasonable that an activated immune system induced by infection could be associated with the development of SJS/TEN and DILI. Ban et al. have reported that acetaminophen can trigger SJS/TEN in patients with suspected infections and T-cells and monocytes may be the key components [6]. It has been suggested that the stimulation or inhibition of cytochrome P450 caused by antibiotics may induce immunological liver damage [27].
Our findings indicated that there were negative associations between anti-infectious drugs and DILD or rhabdomyolysis. DILD and rhabdomyolysis had higher proportion of cases with non-infection drug compared to SJS/ TEN or DILI. This association seems to be caused by the high proportion of cases with non-infection drug, rather than protective effects of infection. Additionally, when we analyzed the associations in the drugs most frequently reported for DILD or rhabdomyolysis, no significant negative associations were observed. Rhabdomyolysis is therefore likely to be caused by drugs other than anti-infectious drugs, especially statins. Potential mechanisms that have been reported include the intracellular depletion of essential metabolites (isoprenoids), destabilization of cell membranes, and the inhibition of organic anion-transporting polypeptide (OATP) 1B1 activity [28,29,30]. Although the contribution of antibodies produced by the statin target, 3-hydroxy-3-methylglutaryl coenzyme A reductase, to the development of severe rhabdomyolysis has been demonstrated, this immune-mediated incidence rate was reported to be very rare [31]. Hence, the immune response is less likely to contribute to the onset of the majority of cases of rhabdomyolysis. Meanwhile, it is thought that a cytotoxic or an immune-mediated reaction might be involved in the development of DILD, but the pathogenesis is not yet fully understood [3]. As results of our analysis, the contribution of immune activation appears to be relatively low in the development of DILD.
To the best of our knowledge, this is the first pharmaco-epidemiological study to examine the associations between anti-infectious drugs and severe ADRs using the spontaneous reporting database in Japan. Since the sample size of spontaneous reporting database is large, it is suitable to analyze severe and/or rarely occurring ADRs. However, we must take into account some limitations when using spontaneous reporting adverse reaction databases. It is difficult to establish causality in a spontaneous reporting database alone. These biases including underreporting, potential biases, misclassification of cases not validated by diagnostic criteria of the ADRs, a lack of information on the number of patients with drug exposure, and insufficient report quality tend to lead to an incorrect result. Although missing data exists in our study, differences in the proportion of missing data among three categories appear relatively small in most of variables but there is the difference in seriousness of ADRs. Similar results to the association between infection and ADR were observed. However, missing data in seriousness of ADRs might have distorted our results. Because patients commonly are prescribed several drugs, it might be incorrect whether anti-infective drug is truly a suspected drug. However, the misclassification is non-differential, and the effect of this misclassification on the results of this study will therefore be limited. Possible effects of reporter and report type might have influenced our findings. However, when restricted to reports by doctor or spontaneous reports, our findings did not change. Moreover, we found a negative association between infection and rhabdomyolysis or DILD. Differences in classification strategies or misclassification regarding drug or infectious disease might have occurred. However, it is assumed that all reported cases seem to have the same probability of being misclassified. These non-differential misclassifications may have led to underestimation of our results.
Furthermore, we should consider a few limitations of this study. First, we could not distinguish whether the associations were caused by the infection itself or were a direct consequence of the anti-infectious drug. However, when we analyzed the association between severe ADRs and concomitant anti-infectious drugs or infectious diseases separately, the same positive associations were observed (data not shown). Thus, our findings may suggest a substantial role of the infection status itself. Second, the severity or type of infectious disease also might affect these ADRs and the effects might vary according to ADRs. With the current data, however, it was not possible to sub-classify causes of infections causes (viral, bacterial, or fungal causes). Third, other potential contributing factors that were not recorded in the existing ADR reports were not evaluated in this study. Fourth, regarding drug classification, although JSCC codes were not completely compatible with ATC codes, which are internationally available, we used the JSCC system for analysis because this system more appropriately reflected clinical indications in Japan. In order to confirm our current findings, further studies that overcome the aforementioned limitations using another database, including electronic medical records, should be conducted.
In summary, we examined the association between infection and four severe ADRs using the spontaneous reporting database in Japan. The results showed that onset of SJS/TEN and DILI was positively associated with infection. Moreover, infection was associated with seriousness of SJS/TEN. In contrast, there was no significant association between infection and DILD or rhabdomyolysis. Thus, this study suggested that infection plays an important role in the development of SJS/TEN and DILI but a less important role for DILD and rhabdomyolysis. Although the limitations of spontaneous reporting systems should be taken into account, for the patients with infection and/or anti-infectious drug, careful monitoring for severe ADRs, especially SJS/TEN, might be needed.
References
Rawlins MD (1981) Clinical pharmacology. Adverse reactions to drugs. BMJ 282
Kubo K, Azuma A, Kanazawa M et al (2013) Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig 51:260–277. doi:10.1016/j.resinv.2013.09.001
Matsuno O (2012) Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 13:39. doi:10.1186/1465-9921-13-39
Amend KL, Landon J, Thyagarajan V et al (2011) Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population. Ann Pharmacother 45:1230–1239. doi:10.1345/aph.1Q110
Thompson PD, Clarkson P, Karas RH (2003) Statin-associated myopathy. JAMA 289:1681. doi:10.1001/jama.289.13.1681
Ban G-Y, Ahn S-J, Yoo H-S et al (2016) Stevens–Johnson syndrome and toxic epidermal necrolysis associated with acetaminophen use during viral infections. Immune Netw 16:256. doi:10.4110/in.2016.16.4.256
Pandit A, Sachdeva T, Bafna P (2011) Drug-induced hepatotoxicity: a review. J Appl Pharm Sci 2:233–243. doi:10.7324/JAPS.2012.2541
Uetrecht J (2007) Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol 47:513–539. doi:10.1146/annurev.pharmtox.47.120505.105150
Neuman MG, Cohen LB, Nanau RM (2015) Quinolones-induced hypersensitivity reactions. Clin Biochem 48:716–739. doi:10.1016/j.clinbiochem.2015.04.006
Tverdek FP, Kofteridis D, Kontoyiannis DP (2016) Antifungal agents and liver toxicity: a complex interaction. Expert Rev Anti-Infect Ther 14:765–776. doi:10.1080/14787210.2016.1199272
Kato Y, Fujii T, Mizoguchi N et al (2000) Potential interaction between ritonavir and carbamazepine. Pharmacotherapy 20:851–854
Lee N-R, Jang JW, Kim HS, Yhim H-Y (2015) Imatinib mesylate-induced interstitial lung disease in a patient with prior history of Mycobacterium tuberculosis infection. Korean J Intern Med 30:550–553. doi:10.3904/kjim.2015.30.4.550
Botnaru V, Munteanu O, Rusu D (2015) Interstitial pneumonitis after treatment for hepatitis C virus infection. Pneumologia 64:46–50
Koubar SH, Estrella MM, Warrier R et al (2017) Rhabdomyolysis in an HIV cohort: epidemiology, causes and outcomes. BMC Nephrol 18:242. doi:10.1186/s12882-017-0656-9
Letko E, Papaliodis DN, Papaliodis GN et al (2005) Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol 94:419–436. doi:10.1016/S1081-1206(10)61112-X
Mockenhaupt M (2011) The current understanding of Stevens–Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol 7:803–815. doi:10.1586/eci.11.66
Lomtadze N, Kupreishvili L, Salakaia A et al (2013) Hepatitis C virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS One 8:e83892. doi:10.1371/journal.pone.0083892
Fernández-Villar A, Sopeña B, García J et al (2007) Hepatitis C virus RNA in serum as a risk factor for isoniazid hepatotoxicity. Infection 35:295–297. doi:10.1007/s15010-007-6125-9
Kwon H, Lee S-H, Kim S-E et al (2012) Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci 27:268. doi:10.3346/jkms.2012.27.3.268
Pichler WJ, Hausmann O (2016) Classification of drug hypersensitivity into allergic, p-i, and pseudo-allergic forms. Int Arch Allergy Immunol 171:166–179. doi:10.1159/000453265
Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK (1998) Adverse drug reactions. BMJ 316:1295–1298
Illing PT, Vivian JP, Purcell AW et al (2013) Human leukocyte antigen-associated drug hypersensitivity. Curr Opin Immunol 25:81–89. doi:10.1016/j.coi.2012.10.002
Böhm R, Cascorbi I (2016) Pharmacogenetics and predictive testing of drug hypersensitivity reactions. Front Pharmacol 7:396. doi:10.3389/fphar.2016.00396
Kaniwa N, Saito Y (2013) Pharmacogenomics of severe cutaneous adverse reactions and drug-induced liver injury. J Hum Genet 58:317–326. doi:10.1038/jhg.2013.37
Usui T, Naisbitt DJ (2017) Human leukocyte antigen and idiosyncratic adverse drug reactions. Drug Metab Pharmacokinet 32:21–30. doi:10.1016/j.dmpk.2016.11.003
Talay O, Shen C-H, Chen L, Chen J (2009) B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci U S A 106:2741–2746
Leitner JM, Graninger W, Thalhammer F (2010) Hepatotoxicity of antibacterials: pathomechanisms and clinical data. Infection 38:3–11. doi:10.1007/s15010-009-9179-z
Evans M, Rees A (2002) Effects of HMG-CoA reductase inhibitors on skeletal muscle: are all statins the same? Drug Saf 25:649–663
Sakamoto K, Kimura J (2013) Mechanism of statin-induced rhabdomyolysis. J Pharmacol Sci 123:289–294. doi:10.1254/jphs.13R06CP
Moßhammer D, Schaeffeler E, Schwab M, Mörike K (2014) Mechanisms and assessment of statin-related muscular adverse effects. Br J Clin Pharmacol 78:454–466. doi:10.1111/bcp.12360
Mammen AL, Tiniakou E (2015) Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 373:1680–1682. doi:10.1056/NEJMc1506163
Acknowledgements
The study was partly supported by a grant (the Research on Regulatory Science of Pharmaceuticals and Medical Devices) from the Japan Agency for Medical Research and Development (AMED) and a Japan Society for the Promotion of Science (JSPS) KAKENHI grant (16K08433).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Imatoh, T., Sai, K., Fukazawa, C. et al. Association between infection and severe drug adverse reactions: an analysis using data from the Japanese Adverse Drug Event Report database. Eur J Clin Pharmacol 73, 1643–1653 (2017). https://doi.org/10.1007/s00228-017-2320-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2320-5