Abstract
Purpose
The aims of this study were to investigate the relationship between metformin exposure, renal clearance (CLR), and apparent non-renal clearance of metformin (CLNR/F) in patients with varying degrees of kidney function and to develop dosing recommendations.
Methods
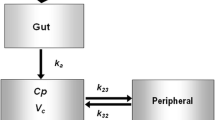
Plasma and urine samples were collected from three studies consisting of patients with varying degrees of kidney function (creatinine clearance, CLCR; range, 14–112 mL/min). A population pharmacokinetic model was built (NONMEM) in which the oral availability (F) was fixed to 0.55 with an estimated inter-individual variability (IIV). Simulations were performed to estimate AUC0-τ, CLR, and CLNR/F.
Results
The data (66 patients, 327 observations) were best described by a two-compartment model, and CLCR was a covariate for CLR. Mean CLR was 17 L/h (CV 22%) and mean CLNR/F was 1.6 L/h (69%).The median recovery of metformin in urine was 49% (range 19–75%) over a dosage interval. When CLR increased due to improved renal function, AUC0-τ decreased proportionally, while CLNR/F did not change with kidney function. Target doses (mg/day) of metformin can be reached using CLCR/3 × 100 to obtain median AUC0–12 of 18–26 mg/L/h for metformin IR and AUC0–24 of 38–51 mg/L/h for metformin XR, with Cmax < 5 mg/L.
Conclusions
The proposed dosing algorithm can be used to dose metformin in patients with various degrees of kidney function to maintain consistent drug exposure. However, there is still marked IIV and therapeutic drug monitoring of metformin plasma concentrations is recommended.





Similar content being viewed by others
References
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359(15):1577–1589. doi:10.1056/NEJMoa0806470
Chen YC, Kok VC, Chien CH, Horng JT, Tsai JJ (2015) Cancer risk in patients aged 30 years and above with type 2 diabetes receiving antidiabetic monotherapy: a cohort study using metformin as the comparator. Ther Clin Risk Manag 11:1315–1323. doi:10.2147/TCRM.S91513
Duong JK, Furlong TJ, Roberts DM, Graham GG, Greenfield JR, Williams KM, Day RO (2013) The role of metformin in metformin-associated lactic acidosis (MALA): case series and formulation of a model of pathogenesis. Drug Saf. doi:10.1007/s40264-013-0038-6
Duong JK, Roberts DM, Furlong TJ, Kumar SS, Greenfield JR, Kirkpatrick CM, Graham GG, Williams KM, Day RO (2012) Metformin therapy in patients with chronic kidney disease. Diabetes Obes Metab. doi:10.1111/j.1463-1326.2012.01617.x
Lalau JD, Lemaire-Hurtel AS, Lacroix C (2011) Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Investig 31(6):435–438. doi:10.2165/11588310-000000000-00000
Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, Lee TC, Timmins P, Graham GG, Furlong TJ, Greenfield JR, Williams KM, Day RO (2013) Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet 52(5):373–384. doi:10.1007/s40262-013-0046-9
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50(2):81–98. doi:10.2165/11534750-000000000-00000
Timmins P, Donahue S, Meeker J, Marathe P (2005) Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clin Pharmacokinet 44(7):721–729. doi:10.2165/00003088-200544070-00004
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed 79(3):241–257. doi:10.1016/j.cmpb.2005.04.005
Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD (2011) Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Prog Biomed 101(1):72–79. doi:10.1016/j.cmpb.2010.04.018
R Core Team (2015). R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. URL http://www.R-project.org/.
Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF (1981) Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol 12(2):235–246
Karlsson MO, Beal SL, Sheiner LB (1995) Three new residual error models for population PK/PD analyses. J Pharmacokinet Biopharm 23(6):651–672
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B (2005) Quantification of lean bodyweight. Clin Pharmacokinet 44(10):1051–1065. doi:10.2165/00003088-200544100-00004
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Janmahasatian S, Duffull SB, Chagnac A, Kirkpatrick CM, Green B (2008) Lean body mass normalizes the effect of obesity on renal function. Br J Clin Pharmacol 65(6):964–965. doi:10.1111/j.1365-2125.2008.03112.x
Karlsson MO, Holford N (2008) A tutorial on visual predictive checks. PAGE. Abstr 1434 [www.page-meeting.org/?abstract=1434]
Henderson AR (2005) The bootstrap: a technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin Chim Acta 359(1–2):1–26. doi:10.1016/j.cccn.2005.04.002
Australian Medicines Handbook (2017) Adelaide: Australian Medicines Handbook Pty Ltd
Savic RM, Karlsson MO (2009) Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11(3):558–569. doi:10.1208/s12248-009-9133-0
Pentikainen PJ, Neuvonen PJ, Penttila A (1979) Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol 16(3):195–202
Choi YH, Lee MG (2006) Effects of enzyme inducers and inhibitors on the pharmacokinetics of metformin in rats: involvement of CYP2C11, 2D1 and 3A1/2 for the metabolism of metformin. Br J Pharmacol 149(4):424–430. doi:10.1038/sj.bjp.0706875
Acknowledgements
This work was performed using computing infrastructure provided by the Australian Centre of Pharmacometrics. The Australian Centre for Pharmacometrics is an initiative of the Australian Government as part of the National Collaborative Research Infrastructure Strategy.
Author information
Authors and Affiliations
Contributions
JKD designed and conducted studies 1 and 2, analysed the data, built the model and wrote the manuscript. MK conducted study 3, analysed the data and contributed to the manuscript. SSK and CMK provided advice on the population model and interpretation of the results. GG was involved in detailed discussions on the clinical significance of the results with JKD. All authors reviewed the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Informed consent was obtained from all individual participants included in the study. Studies 1 and 2 were approved by the Human Research Ethics Committee at St. Vincent’s Hospital and University of New South Wales, Sydney (08209/SVH08/035; 09280/SVH09/080), and were registered with the Australian New Zealand Clinical Trials Registry (ACTRN12611000908932). Study 3 was approved by the Ethics Committee of the University Medical Center Groningen (Almelo, The Netherlands; METc 2013.178).
Funding
This study was funded by the NH&MRC Program Grant 568612, Australian Research Council Grant LP 0990670 and St Vincent’s Clinic Foundation Sister Mary Bernice Research Grant. JKD and SSK had support from the Australian Research Council (ARC) Linkage Grant LP 0990670 for the submitted work.
Competing interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Duong, J.K., Kroonen, M.Y.A.M., Kumar, S.S. et al. A dosing algorithm for metformin based on the relationships between exposure and renal clearance of metformin in patients with varying degrees of kidney function. Eur J Clin Pharmacol 73, 981–990 (2017). https://doi.org/10.1007/s00228-017-2251-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2251-1