Abstract
Purpose
We proposed a statistical criterion to detect drug-drug interactions causing adverse drug reactions in spontaneous reporting systems.
Methods
The used criterion quantitatively measures the discrepancy between the observed and expected number of adverse events via chi-square statistics. We compared the performance of our method with that of Norén et al. (Stat Med 2008; 27 (16): 3057–3070) through a simulation study.
Results
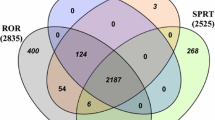
When the number of events for a combination of two drugs was equal to or lower than two, the false positive rate for our method ranged from 0.01 to 0.08, whereas the rate for Norén’s method ranged from 0.01 to 0.06. The sensitivity for our method ranged from 0.09 to 0.29, whereas the sensitivity for Norén’s method ranged from 0.03 to 0.24. The area-under-the-receiver operating characteristic curve for our method was significantly larger than that for Norén’s methods regardless of simulation settings. The proposed method was also applied to the Food and Drug Administration Adverse Event Reporting System database, and a recognized drug-drug interaction was detected.
Conclusions
The proposed criterion controlled false positives at an acceptable level and had higher sensitivity than that of Norén’s method had when events were rare.


Similar content being viewed by others
References
Bushardt RL, Massey EB, Simpson TW et al (2008) Polypharmacy: misleading, but manageable. Clin Interv Aging 3:383
Strandell MJ, Caster O, Bate A et al (2011) Reporting patterns indicative of adverse drug interactions: a systematic evaluation in VigiBase. Drug Saf 34:253–266
Baxter K (ed) (2006) Stockley’s drug interactions, 7th edn. Pharmaceutical Press, London
van Puijenbroek EP, Egberts AC, Meyboom RH et al (1999) Signalling possible drug–drug interactions in a spontaneous reporting system: delay of withdrawal bleeding during concomitant use of oral contraceptives and itraconazole. Br J Clin Pharmacol 47:689–693
van Puijenbroek EP, Egberts AC, Heerdink ER et al (2000) Detecting drug–drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol 56:733–738
Iyer SV, Harpaz R, LePendu P et al (2014) Mining clinical text for signals of adverse drug-drug interactions. J Am Med Inform Assoc 21:353–362
Thakrar BT, Grundschober SB, Doessegger L (2007) Detecting signals of drug–drug interactions in a spontaneous reports database. Br J Clin Pharmacol 64:489–495
Norén GN, Bate A, Orre R et al (2006) Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat Med 25:3740–3757
Bate A, Lindquist M, Edwards IR et al (1998) A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 54:315–321
Norén GN, Sundberg R, Bate A et al (2008) A statistical methodology for drug–drug interaction surveillance. Stat Med 27:3057–3070
Strandell J, Caster O, Bate A et al (2010) 56. Reporting patterns indicative of emerging drug interactions. Drug Saf 33
Qian Y, Ye X, Du W et al (2010) A computerized system for detecting signals due to drug–drug interactions in spontaneous reporting systems. Br J Clin Pharmacol 6:67–73
Strandell J, Caster O, Hopstadius J et al (2013) The development and evaluation of triage algorithms for early discovery of adverse drug interactions. Drug Saf 36:371–388
Norén GN, Hopstadius J, Bate A (2013) Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res 22:57–69
Yates F (1934) Contingency tables involving small numbers and the χ2 test. Suppl J R Stat Soc 1:217–235
Graham DJ, Staffa JA, Shatin D et al (2004) Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 292:2585–2590
Wilson PW, Kannel WB, Anderson KM (1985) Lipids, glucose intolerance and vascular disease: the Framingham study. Monogr Atheroscler 13:1
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies. J Natl Cancer Inst 22:719–748
Gould L (2003) Practical pharmacovigilance analysis strategies. Pharmacoepidemiol Drug Saf 12:559–574
Bate A, Evans SW (2009) Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 18:427–436
Hopstadius J, Norén GN, Bate A (2008) Impact of stratification on adverse drug reaction surveillance. Drug Saf 31:1035–1048
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number 15K19219.
Contributions of authors
M.G. was responsible for study concept, analysis and interpretation of results, and wrote the first draft of the manuscript. K.M. and A.H. interpreted the study results. K.T. reviewed the simulation code. All authors elaborated and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 176 kb)
Rights and permissions
About this article
Cite this article
Gosho, M., Maruo, K., Tada, K. et al. Utilization of chi-square statistics for screening adverse drug-drug interactions in spontaneous reporting systems. Eur J Clin Pharmacol 73, 779–786 (2017). https://doi.org/10.1007/s00228-017-2233-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2233-3