Abstract
Objectives
The aim of this study was to evaluate different dosage regimens of meropenem in elderly patients in relation with renal function using a population pharmacokinetic (popPK) model.
Methods
The data of 178 elderly patients treated with meropenem was collected from different sources. A popPK model was developed by using NONMEM® and the influence of different covariates on meropenem CL and V1 was observed. Monte Carlo dosing simulations were performed at steady state to observe the % T > MIC for targets of 40, 60 and 80% of dosage intervals at different levels of creatinine clearance (CLCR).
Results
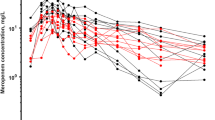
The data was described by a two-compartment model and the values of parameter estimates for CL, V1, Q and V2 were 5.27 L/h, 17.2 L, 9.92 L/h and 10.6 L, respectively. The CLCR, body weight and centre had a significant influence on meropenem CL while no direct influence of age was observed. Extended infusions had pharmacokinetic and pharmacodynamic (PK/PD) breakpoint one dilution greater than corresponding short infusion regimens for each target of % T > MIC.
Conclusion
Meropenem CL was significantly lower in the elderly compared to CL reported in younger patients due to the reduced renal function. An extended infusion of 1000 mg q8h can be considered for empirical treatment of infections in elderly patients when CLCR is ≤ 50 mL/min. A continuous infusion of 3000 mg daily dose is preferred if CLCR > 50 mL/min. However, a higher daily dose of meropenem would be required for resistant strains (MIC >8 mg/L) of bacteria if CLCR is >100 mL/min.




Similar content being viewed by others
References
Wiseman LR et al (1995) Meropenem. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 50(1):73–101
Spellberg B et al (2011) Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 52(Suppl 5):S397–S428
Boucher HW et al (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48(1):1–12
Wittau, M., et al. 2015. Population pharmacokinetics and target attainment of meropenem in plasma and tissue of morbidly obese patients after laparoscopic intraperitoneal surgery. Antimicrob Agents Chemother
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26(1):1–10 quiz 11-2
Nicolau DP (2008) Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis 47(Suppl 1):S32–S40
Shekar K et al (2014) The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit Care 18(6):565
Li C et al (2007) Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51(5):1725–1730
De Waele JJ et al (2014) Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med 40(3):380–387
Ulldemolins, M., et al. 2015 Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother
Mouton JW, van den Anker JN (1995) Meropenem clinical pharmacokinetics. Clin Pharmacokinet 28(4):275–286
Udy AA et al (2012) Subtherapeutic initial beta-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 142(1):30–39
Taccone FS et al (2010) Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 14(4):R126
Varghese JM, Roberts JA, Lipman J (2011) Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit Care Clin 27(1):19–34
Taccone FS et al (2011) Appropriate antibiotic dosage levels in the treatment of severe sepsis and septic shock. Curr Infect Dis Rep 13(5):406–415
Blanchet B et al (2008) Influence of burns on pharmacokinetics and pharmacodynamics of drugs used in the care of burn patients. Clin Pharmacokinet 47(10):635–654
Beumier M et al (2015) Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol 81(5):497–506
Verhave JC et al (2005) Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis 46(2):233–241
Jaruratanasirikul S et al (2015) Population pharmacokinetics and Monte Carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units. Antimicrob Agents Chemother 59(6):2995–3001
Goncalves-Pereira J et al (2014) Assessment of pharmacokinetic changes of meropenem during therapy in septic critically ill patients. BMC Pharmacol Toxicol 15:21
Ramon-Lopez A et al (2015) Dosing regimen of meropenem for adults with severe burns: a population pharmacokinetic study with Monte Carlo simulations. J Antimicrob Chemother 70(3):882–890
Doh K et al (2010) Population pharmacokinetics of meropenem in burn patients. J Antimicrob Chemother 65(11):2428–2435
Isla A et al (2008) Population pharmacokinetics of meropenem in critically ill patients undergoing continuous renal replacement therapy. Clin Pharmacokinet 47(3):173–180
Jamal JA et al (2015) Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: a randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents 45(1):41–45
Kees, M.G., et al. 2015 Population pharmacokinetics of meropenem during continuous infusion in surgical ICU patients. J Clin Pharmacol
Alobaid, A.S., et al. 2015 What is the effect of obesity on piperacillin and meropenem trough concentrations in critically ill patients? J Antimicrob Chemother
Bias M, Frey O, Köberer A (2010) HPLC-Methode zur quantitativen Bestimmung von Meropenem im Serum. Krankenhauspharmazie 31(11):482–485
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed 79(3):241–257
Keizer RJ et al (2011) Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Prog Biomed 101(1):72–79
European Committee of Antimicrobial Susceptibility testing (EUCAST) 2016 Clinical breakpoints. [cited 2016 28 Feb 2016]; Available from: http://www.eucast.org/clinical_breakpoints
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Devine BJ (1974) Gentamicin therapy. Drug Intell Clin Pharm 8:650–655
Zhou QT et al (2011) Pharmacokinetics and pharmacodynamics of meropenem in elderly chinese with lower respiratory tract infections: population pharmacokinetics analysis using nonlinear mixed-effects modelling and clinical pharmacodynamics study. Drugs Aging 28(11):903–912
Krueger WA et al (2005) Evaluation by Monte Carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healthy volunteers. Antimicrob Agents Chemother 49(5):1881–1889
Roberts JA et al (2009) Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64(1):142–150
Lodise TP et al (2007) Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J Antimicrob Chemother 60(5):1038–1044
Li C et al (2006) Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol 46(10):1171–1178
Ariano RE et al (2005) Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann Pharmacother 39(1):32–38
Christensson BA et al (1992) Pharmacokinetics of meropenem in subjects with various degrees of renal impairment. Antimicrob Agents Chemother 36(7):1532–1537
Ikawa K et al (2010) Population pharmacokinetics and pharmacodynamics of meropenem in Japanese pediatric patients. J Infect Chemother 16(2):139–143
Shino N et al (2015) Development and assessment of a nomogram to propose the initial dosage regimen of a meropenem infusion based on serum creatinine and age using a Monte Carlo simulation. Chem Pharm Bull (Tokyo) 63(12):986–991
Frippiat F et al (2015) Modelled target attainment after meropenem infusion in patients with severe nosocomial pneumonia: the PROMESSE study. J Antimicrob Chemother 70(1):207–216
Lorente L et al (2006) Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother 40(2):219–223
Berthoin K et al (2010) Stability of meropenem and doripenem solutions for administration by continuous infusion. J Antimicrob Chemother 65(5):1073–1075
Acknowledgement
We are highly thankful to Dr. Arantxazu Isla and Dr. Dong-Seok Yim for providing us the meropenem data of elderly patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The medical ethics committees of the respective centres approved collection of samples. Moreover, sample collection was in accordance with good clinical practices as described in Helsinki Declaration of Good Clinical Practice guidelines.
Conflict of interest
The Higher Education Commission (HEC) of Pakistan supported MU financially via the German Academic Exchange Service (DAAD) Germany. Funding program number 50015451.
OF and GH have no conflict of interest for conducting this study.
Electronic supplementary material
Supplementary file S1
(DOCX 12 kb).
Supplementary file S2
(DOCX 17 kb).
Rights and permissions
About this article
Cite this article
Usman, M., Frey, O.R. & Hempel, G. Population pharmacokinetics of meropenem in elderly patients: dosing simulations based on renal function. Eur J Clin Pharmacol 73, 333–342 (2017). https://doi.org/10.1007/s00228-016-2172-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2172-4