Abstract
Purpose
The majority of angiotensin-converting enzyme inhibitors (ACEIs) are synthesized as ester prodrugs that must be converted to their active forms in vivo in order to exert therapeutic effects. Hepatic carboxylesterase 1 (CES1) is the primary enzyme responsible for the bioactivation of ACEI prodrugs in humans. The genetic variant −816A>C (rs3785161) is a common variant located in the promoter region of the CES1P1 gene. Previous studies report conflicting results with regard to the association of this variant and therapeutic outcomes of CES1 substrate drugs. The purpose of this study was to determine the effect of the variant −816A>C on the activation of the ACEI prodrug trandolapril in human livers and the blood pressure (BP)-lowering effect of trandolapril in hypertensive patients.
Methods
The −816A>C genotypes and CES1 expression and activity on trandolapril activation were determined in 100 individual human liver samples. Furthermore, the association of the −816A>C variant and the BP lowering effect of trandolapril was evaluated in hypertensive patients who participated in the International Verapamil SR Trandolapril Study (INVEST).
Results
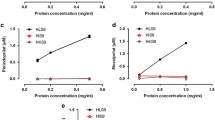
Our in vitro study demonstrated that hepatic CES1 expression and activity did not differ among different −816A>C genotypes. Moreover, we were unable to identify a clinical association between the BP lowering effects of trandolapril and −816A>C genotypes.
Conclusions
We conclude that the −816A>C variant is not associated with interindividual variability in CES1 expression and activity or therapeutic response to ACEI prodrugs.



Similar content being viewed by others
References
Takai S, Matsuda A, Usami Y, Adachi T, Sugiyama T, Katagiri Y, Tatematsu M, Hirano K (1997) Hydrolytic profile for ester- or amide-linkage by carboxylesterases pI 5.3 and 4.5 from human liver. Biol Pharm Bull 20(8):869–873
Zhu HJ, Appel DI, Johnson JA, Chavin KD, Markowitz JS (2009) Role of carboxylesterase 1 and impact of natural genetic variants on the hydrolysis of trandolapril. Biochem Pharmacol 77(7):1266–1272. doi:10.1016/j.bcp.2008.12.017
Thomsen R, Rasmussen HB, Linnet K (2013) In vitro drug metabolism by human carboxylesterase 1: focus on angiotensin-converting enzyme inhibitors. Drug Metab Dispos. doi:10.1124/dmd.113.053512
Wang X, Wang G, Shi J, Aa J, Comas R, Liang Y, Zhu HJ (2015) CES1 genetic variation affects the activation of angiotensin-converting enzyme inhibitors. Pharmacogenomics J. doi:10.1038/tpj.2015.1042 tpj201542
Geshi E, Kimura T, Yoshimura M, Suzuki H, Koba S, Sakai T, Saito T, Koga A, Muramatsu M, Katagiri T (2005) A single nucleotide polymorphism in the carboxylesterase gene is associated with the responsiveness to imidapril medication and the promoter activity. Hypertens Res 28(9):719–725
Xie C, Ding X, Gao J, Wang H, Hang Y, Zhang H, Zhang J, Jiang B, Miao L (2014) The effects of CES1A2 A(-816)C and CYP2C19 loss-of-function polymorphisms on clopidogrel response variability among Chinese patients with coronary heart disease. Pharmacogenet Genomics. doi:10.1097/FPC.0000000000000035
Zou J-J, Chen S-L, Fan H-W, Tan J, He B-S, Xie H-G (2014) The CES1A -816C as a genetic marker to predict greater platelet clopidogrel response in patients with percutaneous coronary intervention. J Cardiovasc Pharmacol 63(2):178–183
Pepine CJ, Handberg-Thurmond E, Marks RG, Conlon M, Cooper-DeHoff R, Volkers P, Zellig P (1998) Rationale and design of the International Verapamil SR/Trandolapril Study (INVEST): an Internet-based randomized trial in coronary artery disease patients with hypertension. J Am Coll Cardiol 32(5):1228–1237
Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW (2003) A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. Jama 290(21):2805–2816
Zhu HJ, Appel DI, Jiang Y, Markowitz JS (2009) Age- and sex-related expression and activity of carboxylesterase 1 and 2 in mouse and human liver. Drug Metab Dispos 37(9):1819–1825. doi:10.1124/dmd.109.028209
Nirogi RV, Kandikere VN, Shrivastava W, Mudigonda K (2006) Quantification of trandolapril and its metabolite trandolaprilat in human plasma by liquid chromatography/tandem mass spectrometry using solid-phase extraction. Rapid Commun Mass Spectrom 20(24):3709–3716. doi:10.1002/rcm.2794
Tsoukas G, Anand S, Yang K (2011) Dose-dependent antihypertensive efficacy and tolerability of perindopril in a large, observational, 12-week, general practice-based study. Am J Cardiovasc Drugs 11(1):45–55. doi:10.2165/11587000-000000000-00000
Malacco E, Omboni S, Volpe M, Auteri A, Zanchetti A (2010) Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens 28(11):2342–2350. doi:10.1097/HJH.0b013e32833e116b
Ionescu DD (2009) Antihypertensive efficacy of perindopril 5-10 mg/day in primary health care: an open-label, prospective, observational study. Clin Drug Investig 29(12):767–776. doi:10.2165/11319700-000000000-00000
Mugellini A, Dobovisek J, Planinc D, Cremonesi G, Fogari R (2004) Efficacy and safety of delapril plus manidipine compared with enalapril plus hydrochlorothiazide in mild to moderate essential hypertension: results of a randomized trial. Clin Ther 26(9):1419–1426. doi:10.1016/j.clinthera.2004.09.018
Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E (2004) Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 17(2):103–111
Poirier L, Bourgeois J, Lefebvre J, Archambault F, Lacourciere Y (1995) ACE inhibitors as first-line treatment agents: a comparative study of trandolapril and enalapril on casual and ambulatory blood pressures. Am J Ther 2(3):159–164
Zhu HJ, Wang X, Gawronski BE, Brinda BJ, Angiolillo DJ, Markowitz JS (2013) Carboxylesterase 1 as a determinant of clopidogrel metabolism and activation. J Pharmacol Exp Ther 344(3):665–672. doi:10.1124/jpet.112.201640 jpet.112.201640
Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, Malcolm R, Johnson JA, Youngblood GL, Sweet DH, Langaee TY, Markowitz JS (2008) Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet 82 (6): 1241–1248 doi:10.1016/j.ajhg.2008.04.015
Zhu HJ, Markowitz JS (2009) Activation of the antiviral prodrug oseltamivir is impaired by two newly identified carboxylesterase 1 variants. Drug Metab Dispos 37(2):264–267. doi:10.1124/dmd.108.024943
Bruxel EM, Salatino-Oliveira A, Genro JP, Zeni CP, Polanczyk GV, Chazan R, Rohde LA, Hutz MH (2012) Association of a carboxylesterase 1 polymorphism with appetite reduction in children and adolescents with attention-deficit/hyperactivity disorder treated with methylphenidate. Pharmacogenomics J. doi:10.1038/tpj.2012.25 tpj201225
Shi D, Yang D, Prinssen EP, Davies BE, Yan B (2011) Surge in expression of carboxylesterase 1 during the post-neonatal stage enables a rapid gain of the capacity to activate the anti-influenza prodrug oseltamivir. J Infect Dis 203(7):937–942. doi:10.1093/infdis/jiq145
Yang D, Pearce RE, Wang X, Gaedigk R, Wan YJ, Yan B (2009) Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol 77(2):238–247. doi:10.1016/j.bcp.2008.10.005
Zhu HJ, Appel DI, Peterson YK, Wang ZC, Markowitz JS (2010) Identification of selected therapeutic agents as inhibitors of carboxylesterase 1: potential sources of metabolic drug interactions. Toxicology 270(2–3):59–65. doi:10.1016/j.tox.2010.01.009
Hatfield MJ, Potter PM (2011) Carboxylesterase inhibitors. Expert Opin Ther Pat. doi:10.1517/13543776.2011.586339
Shi D, Yang J, Yang D, LeCluyse EL, Black C, You L, Akhlaghi F, Yan B (2006) Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther 319(3):1477–1484
Rhoades JA, Peterson YK, Zhu HJ, Appel DI, Peloquin CA, Markowitz JS (2011) Prediction and in vitro evaluation of selected protease inhibitor antiviral drugs as inhibitors of carboxylesterase 1: a potential source of drug-drug interactions. Pharm Res. doi:10.1007/s11095-011-0637-9
Kristensen KE, Zhu HJ, Wang X, Gislason GH, Torp-Pedersen C, Rasmussen HB, Markowitz JS, Hansen PR (2014) Clopidogrel bioactivation and risk of bleeding in patients cotreated with angiotensin-converting enzyme inhibitors after myocardial infarction: a proof-of-concept study. Clin Pharmacol Ther 96(6):713–722. doi:10.1038/clpt.2014.183
Tarkiainen EK, Holmberg MT, Tornio A, Neuvonen M, Neuvonen PJ, Backman JT, Niemi M (2015) Carboxylesterase 1 c.428G>A single nucleotide variation increases the antiplatelet effects of clopidogrel by reducing its hydrolysis in humans. Clin Pharmacol Ther 97(6):650–658. doi:10.1002/cpt.101
Yoshimura M, Kimura T, Ishii M, Ishii K, Matsuura T, Geshi E, Hosokawa M, Muramatsu M (2008) Functional polymorphisms in carboxylesterase1A2 (CES1A2) gene involves specific protein 1 (Sp1) binding sites. Biochem Biophys Res Commun 369(3):939–942
Sai K, Saito Y, Tatewaki N, Hosokawa M, Kaniwa N, Nishimaki-Mogami T, Naito M, Sawada J, Shirao K, Hamaguchi T, Yamamoto N, Kunitoh H, Tamura T, Yamada Y, Ohe Y, Yoshida T, Minami H, Ohtsu A, Matsumura Y, Saijo N, Okuda H (2010) Association of carboxylesterase 1A genotypes with irinotecan pharmacokinetics in Japanese cancer patients. Br J Clin Pharmacol 70(2):222–233. doi:10.1111/j.1365-2125.2010.03695.x
Fukami T, Nakajima M, Maruichi T, Takahashi S, Takamiya M, Aoki Y, McLeod HL, Yokoi T (2008) Structure and characterization of human carboxylesterase 1A1, 1A2, and 1A3 genes. Pharmacogenet Genomics 18(10):911–920. doi:10.1097/FPC.0b013e32830b0c5e
Acknowledgments
The research reported in this publication was supported in part by the National Institute on Aging (R21AG048500) (Hao-Jie Zhu), the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 2UL1TR000433 (Hao-Jie Zhu), and the American Association of Colleges of Pharmacy (AACP) 2015 New Investigator Award (Hao-Jie Zhu).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Zhu, HJ., Langaee, T.Y., Gong, Y. et al. CES1P1 variant −816A>C is not associated with hepatic carboxylesterase 1 expression and activity or antihypertensive effect of trandolapril. Eur J Clin Pharmacol 72, 681–687 (2016). https://doi.org/10.1007/s00228-016-2029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2029-x