Abstract
Purpose
Lamotrigine (LTG) is used to treat epilepsy. The variability of LTG pharmacokinetics among individuals may be attributed to polymorphisms in the genes of uridine diphosphate glucuronosyltransferases (UGTs) 1A4 and UGT2B7 and/or combination with other drugs. In this study, we evaluated the association between LTG concentrations and patient characteristics such as genetic polymorphisms and the co-administration of antiepileptic drugs.
Methods
We recruited 122 patients with epilepsy. LTG concentrations were measured in blood samples from each patient under steady-state condition. We assessed the influence of multiple factors on LTG concentrations and derived a formula for predicting LTG concentrations using multiple linear regression analysis.
Results
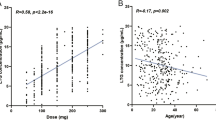
We derived a formula to predict LTG concentrations that considers the daily dose of LTG, body weight, valproic acid concentration, phenytoin co-administration, and the co-administration of phenobarbital and/or carbamazepine as well as UGT1A4 142T>G and UGT2B7 -161C>T polymorphisms (adjusted coefficients of determination R 2 = 0.734). Furthermore, we used this formula to reveal a strong positive correlation between measured and predicted LTG concentrations (r 2 = 0.76, p < 0.001).
Conclusion
We derived a formula that will be useful in clinical practice for predicting LTG concentrations in patients with epilepsy.

Similar content being viewed by others
References
Morris RG, Black AB, Harris AL, Batty AB, Sallustio BC (1998) Lamotrigine and therapeutic drug monitoring: retrospective survey following the introduction of a routine service. Br J Clin Pharmacol 46:547–551
Johannessen SI, Tomson T (2006) Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed? Clin Pharmacokinet 45:1061–1075
Neels HM, Sierens AC, Naelaerts K, Scharpé SL, Hatfield GM, Lambert WE (2004) Therapeutic drug monitoring of old and newer anti-epileptic drugs. Clin Chem Lab Med 42:1228–1255
Johannessen SI, Battino D, Berry DJ, Bialer M, Krämer G, Tomson T, Patsalos PN (2003) Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit 25:347–363
Rowland A, Elliot DJ, Williams JA, Mackenzie PI, Dickinson RG, Miners JO (2006) In vitro characterization of lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metab Dispos 34:1055–1062
Saeki M, Saito Y, Jinno H, Sai K, Hachisuka A, Kaniwa N, Ozawa S, Kawamoto M, Kamatani N, Shirao K, Minami H, Ohtsu A, Yoshida T, Saijo N, Komamura K, Kotake T, Morishita H, Kamakura S, Kitakaze M, Tomoike H, Sawada J (2005) Genetic variations and haplotypes of UGT1A4 in a Japanese population. Drug Metab Pharmacokinet 20:144–151
Gulcebi MI, Ozkaynakcı A, Goren MZ, Aker RG, Ozkara C, Onat FY (2011) The relationship between UGT1A4 polymorphism and serum concentration of lamotrigine in patients with epilepsy. Epilepsy Res 95:1–8
Ishii Y, Hansen AJ, Mackenzie PI (2000) Octamer transcription factor-1 enhances hepatic nuclear factor-1alpha-mediated activation of the human UDP glucuronosyltransferase 2B7 promoter. Mol Pharmacol 57:940–947
Singkham N, Towanabut S, Lertkachatarn S, Punyawudho B (2013) Influence of the UGT2B7 -161C>T polymorphism on the population pharmacokinetics of lamotrigine in Thai patients. Eur J Clin Pharmacol 69:1285–1291
Yamamoto Y, Takahashi Y, Imai K, Ikeda H, Takahashi M, Nakai M, Inoue Y, Kagawa Y (2015) Influence of uridine diphosphate glucuronosyltransferase inducers and inhibitors on the plasma lamotrigine concentration in pediatric patients with refractory epilepsy. Drug Metab Pharmacokinet 30:214–220
Kanner AM, Frey M (2000) Adding valproate to lamotrigine: a study of their pharmacokinetic interaction. Neurology 55:588–591
May TW, Rambeck B, Jürgens U (1996) Serum concentrations of lamotrigine in epileptic patients: the influence of dose and comedication. Ther Drug Monit 18:523–531
Liu L, Zhao L, Wang Q, Qiu F, Wu X, Ma Y (2015) Influence of valproic acid concentration and polymorphism of UGT1A4*3, UGT2B7 -161C>T and UGT2B7*2 on serum concentration of lamotrigine in Chinese epileptic children. Eur J Clin Pharmacol 71:1341–1347
Rickham PP (1964) Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br Med J 2:177
Saeki M, Saito Y, Jinno H, Tanaka-Kagawa T, Ohno A, Ozawa S, Ueno K, Kamakura S, Kamatani N, Komamura K, Kitakaze M, Sawada J (2004) Single nucleotide polymorphisms and haplotype frequencies of UGT2B4 and UGT2B7 in a Japanese population. Drug Metab Dispos 32:1048–1054
Saito K, Moriya H, Sawaguchi T, Hayakawa T, Nakahara S, Goto A, Arimura Y, Imai K, Kurosawa N, Owada E, Miyamoto A (2006) Haplotype analysis of UDP-glucuronocyltransferase 2B7 gene (UGT2B7) polymorphisms in healthy Japanese subjects. Clin Biochem 39:303–308
Blanca Sánchez M, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, Nicolas JM, Adín J, Shushtarian M, Armijo JA (2010) UGT2B7 -161C>T polymorphism is associated with lamotrigine concentration-to-dose ratio in a multivariate study. Ther Drug Monit 32:177–184
Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, Manns MP (2002) Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 50:259–265
Miyagi SJ, Collier AC (2007) Pediatric development of glucuronidation: the ontogeny of hepatic UGT1A4. Drug Metab Dispos 35:1587–1592
Wegner I, Wilhelm AJ, Sander JW, Lindhout D (2013) The impact of age on lamotrigine and oxcarbazepine kinetics: a historical cohort study. Epilepsy Behav 29:217–221
Naik GS, Kodagali R, Mathew BS, Thomas M, Prabha R, Mathew V, Fleming DH (2015) Therapeutic drug monitoring of levetiracetam and lamotrigine: is there a need? Ther Drug Monit 37:437–444
Reimers A, Sjursen W, Helde G, Brodtkorb E (2014) Frequencies of UGT1A4*2 (P24T) and *3 (L48V) and their effects on serum concentrations of lamotrigine. Eur J Drug Metab Pharmacokinet
Wang Q, Liang M, Dong Y, Yun W, Qiu F, Zhao L, Guo Y (2015) Effects of UGT1A4 genetic polymorphisms on serum lamotrigine concentrations in Chinese children with epilepsy. Drug Metab Pharmacokinet 30:209–213
Takekuma Y, Takenaka T, Kiyokawa M, Yamazaki K, Okamoto H, Kitabatake A, Tsutsui H, Sugawara M (2006) Contribution of polymorphisms in UDP-glucuronosyltransferase and CYP2D6 to the individual variation in disposition of carvedilol. J Pharm Pharm Sci 9:101–112
Takekuma Y, Takenaka T, Kiyokawa M, Yamazaki K, Okamoto H, Kitabatake A, Tsutsui H, Sugawara M (2007) Evaluation of effects of polymorphism for metabolic enzymes on pharmacokinetics of carvedilol by population pharmacokinetic analysis. Biol Pharm Bull 30:537–542
Anderson GD (1998) A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother 32:554–563
Patsalos PN, Perucca E (2003) Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol 2:347–356
Yamamoto Y, Inoue Y, Matsuda K, Takahashi Y, Kagawa Y (2012) Influence of concomitant antiepileptic drugs on plasma lamotrigine concentration in adult Japanese epilepsy patients. Biol Pharm Bull 35:487–493
Patsalos PN (2013) Drug interactions with the newer antiepileptic drugs (AEDs)—part 1: pharmacokinetic and pharmacodynamic interactions between AEDs. Clin Pharmacokinet 52:927–966
Johannessen Landmark C, Patsalos PN (2010) Drug interactions involving the new second- and third-generation antiepileptic drugs. Expert Rev Neurother 10:119–140
Perucca E, Cloyd J, Critchley D, Fuseau E (2008) Rufinamide: clinical pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia 49:1123–1141
Reimers A, Skogvoll E, Sund JK, Spigset O (2005) Drug interactions between lamotrigine and psychoactive drugs: evidence from a therapeutic drug monitoring service. J Clin Psychopharmacol 25:342–348
Sidhu J, Job S, Singh S, Philipson R (2006) The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol 61:191–199
Sabers A, Buchholt JM, Uldall P, Hansen EL (2001) Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res 47:151–154
Paragliola RM, Prete A, Kaplan PW, Corsello SM, Salvatori R (2015) Treatment of hypopituitarism in patients receiving antiepileptic drugs. Lancet Diabetes Endocrinol 3:132–140
Sharma C, Dubey R, Kumar H, Saha N (2005) Food reduces the bioavailability of lamotrigine. Indian J Med Res 121:659–664
Kverneland M, Taubøll E, Selmer KK, Iversen PO, Nakken KO (2015) Modified Atkins diet may reduce serum concentrations of antiepileptic drugs. Acta Neurol Scand 131:187–190
Reinsberger C, Dorn T, Krämer G (2008) Smoking reduces serum levels of lamotrigine. Seizure 17:651–653
Shenfield GM (1993) Oral contraceptives. Are drug interactions of clinical significance? Drug Saf 9:21–37
Reimers A (2004) Oral contraceptives can affect the metabolism of other drugs. Tidsskr Nor Laegeforen 124:1785–1787
Zaccara G, Perucca E (2014) Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord 16:409–431
Perucca E (2006) Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 61:246–255
Preskorn SH (1997) Clinically relevant pharmacology of selective serotonin reuptake inhibitors. An overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet 32(Suppl 1):1–21
Pleym H, Spigset O, Kharasch ED, Dale O (2003) Gender differences in drug effects: implications for anesthesiologists. Acta Anaesthesiol Scand 47:241–259
Jawad S, Yuen WC, Peck AW, Hamilton MJ, Oxley JR, Richens A (1987) Lamotrigine: single-dose pharmacokinetics and initial 1 week experience in refractory epilepsy. Epilepsy Res 1:194–201
Cohen AF, Land GS, Breimer DD, Yuen WC, Winton C, Peck AW (1987) Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther 42:535–541
Eriksson AS, Hoppu K, Nergårdh A, Boreus L (1996) Pharmacokinetic interactions between lamotrigine and other antiepileptic drugs in children with intractable epilepsy. Epilepsia 37:769–773
Reimers A (2009) Trends and changes in the clinical use of lamotrigine. Pharmacoepidemiol Drug Saf 18:132–139
Søndergaard Khinchi M, Nielsen KA, Dahl M, Wolf P (2008) Lamotrigine therapeutic thresholds. Seizure 17:391–395
Werz MA (2008) Pharmacotherapeutics of epilepsy: use of lamotrigine and expectations for lamotrigine extended release. Ther Clin Risk Manag 4:1035–1046
Rivas N, Buelga DS, Elger CE, Santos-Borbujo J, Otero MJ, Domínguez-Gil A, García MJ (2008) Population pharmacokinetics of lamotrigine with data from therapeutic drug monitoring in German and Spanish patients with epilepsy. Ther Drug Monit 30:483–489
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Inoue, K., Yamamoto, Y., Suzuki, E. et al. Factors that influence the pharmacokinetics of lamotrigine in Japanese patients with epilepsy. Eur J Clin Pharmacol 72, 555–562 (2016). https://doi.org/10.1007/s00228-016-2008-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2008-2