Abstract
Purpose
The purpose of this study is to investigate the effect of two of the most important functional CYP1A2 variations −3860G > A and −163C > A on carbamazepine pharmacokinetics in Serbian pediatric epileptic patients.
Methods
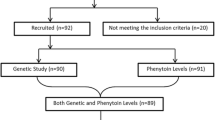
The study involved 40 Serbian pediatric epileptic patients on steady-state carbamazepine treatment. Genotyping for −3860G > A and −163C > A was carried out using PCR-RFLP method, and carbamazepine plasma concentrations were determined by high pressure liquid chromatography (HPLC) method. For pharmacokinetic analysis, NONMEM software with implementation of ADVAN 1 subroutine was used.
Results
CYP1A2 polymorphism −163C > A was found at the frequency of 65.0 %, while −3860G > A was not detected. The correlation between weight-adjusted carbamazepine dose and carbamazepine concentration after dose adjustment was significant only in carriers of −163C/C and C/A genotypes (r = 0.68, p = 0.0004). The equation that described population clearance (CL) was CL (l/h) = 0.176 + 0.0484 * SEX + 0.019 * CYP1A2 + 0.000156 * DD, where SEX has a value of 1 if male and 0 if female, CYP1A2 has a value of 1 if −163A/A and 0 if −163C/C or C/A, and DD is the total carbamazepine daily dose (mg/day).
Conclusions
CYP1A2 −163A/A genotype influence carbamazepine pharmacokinetics. In addition to sex and total carbamazepine daily dose, −163C > A CYP1A2 polymorphism should be considered as a predictor of carbamazepine clearance.

Similar content being viewed by others
References
Thorn CF, Leckband SG, Kelsoe J, Leeder JS, Muller DJ, Klein TE, Altman RB (2011) PharmGKB summary: carbamazepine pathway. Pharmacogenet Genomics 21(12):906–910
Tolou-Ghamari Z, Zare M, Habibabadi JM, Najafi MR (2013) A quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. Res Med Sci 18(1):81–85
Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, Resnick T, Benbadis SR (2014) The consequences of refractory epilepsy and its treatment. Epilepsy Behav 37C:59–70
Löscher W, Klotz U, Zimprich F, Schmidt D (2009) The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia 50(1):1–23
Bertilsson L, Tomson T (1986) Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine-10,11-epoxide. An update. Clin Pharmacokinet 11(3):177–198
Pearce RE, Uetrecht JP, Leeder JS (2005) Pathways of carbamazepine bioactivation in vitro: II. The role of human cytochrome P450 enzymes in the formation of 2-hydroxyiminostilbene. Drug Metab Dispos 33(12):1819–1826
Pearce RE, Vakkalagadda GR, Leeder JS (2002) Pathways of carbamazepine bioactivation in vitro I. Characterization of human cytochromes P450 responsible for the formation of 2- and 3-hydroxylated metabolites. Drug Metab Dispos 30(11):1170–1179
Pearce RE, Lu W, Wang Y, Uetrecht JP, Correia MA, Leeder JS (2008) Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine. Drug Metab Dispos 36(8):1637–1649
Parker AC, Pritchard P, Preston T, Choonara I (1998) Induction of CYP1A2 activity by carbamazepine in children using the caffeine breath test. Br J Clin Pharmacol 45(2):176–178
Lucas RA, Gilfillan DJ, Bergstrom RF (1998) A pharmacokinetic interaction between carbamazepine and olanzapine: observations on possible mechanism. Eur J Clin Pharmacol 54(8):639–643
Oscarson M, Zanger UM, Rifki OF, Klein K, Eichelbaum M, Meyer UA (2006) Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin Pharmacol Ther 80(5):440–456
Magnusson MO, Dahl ML, Cederberg J, Karlsson MO, Sandstrom R (2008) Pharmacodynamics of carbamazepine-mediated induction of CYP3A4, CYP1A2, and Pgp as assessed by probe substrates midazolam, caffeine, and digoxin. Clin Pharmacol Ther 84(1):52–62
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141
Sachse C, Brockmoller J, Bauer S, Roots I (1999) Functional significance of a C→A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 47(4):445–449
Djordjevic N, Ghotbi R, Jankovic S, Aklillu E (2010) Induction of CYP1A2 by heavy coffee consumption is associated with the CYP1A2 − 163C > A polymorphism. Eur J Clin Pharmacol 66:697–703
Jankovic SM, Jovanovic D, Milovanovic JR (2008) Pharmacokinetic modeling of carbamazepine based on clinical data from Serbian epileptic patients. Methods Find Exp Clin Pharmacol 30(9):707–713
Bertilsson L, Hojer B, Tybring G, Osterloh J, Rane A (1980) Autoinduction of carbamazepine metabolism in children examined by a stable isotope technique. Clin Pharmacol Ther 27(1):83–88
Rane A, Hojer B, Wilson JT (1976) Kinetics of carbamazepine and its 10,11-epoxide metabolite in children. Clin Pharmacol Ther 19(3):276–283
Perucca E (1995) Pharmacological problems in the management of epilepsy in children. Seizure 4(2):139–143
Sweetman S (ed) (2009) Martindale: the complete drug reference 36. Pharmaceutical Press, London
Blanco JG, Harrison PL, Evans WE, Relling MV (2000) Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab Dispos 28(4):379–382
Benedetti MS, Whomsley R, Canning M (2007) Drug metabolism in the paediatric population and in the elderly. Drug Discov Today 12(15–16):599–610
Diczfalusy U, Nylén H, Elander P, Bertilsson L (2010) 4b-hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol 71(2):183–189
Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T (1999) Genetic polymorphism in the 5’-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem 125(4):803–808
Milovanovic JR, Jankovic SM (2011) Factors influencing carbamazepine pharmacokinetics in children and adults: population pharmacokinetic analysis. Int J Pharmacol Ther 49(7):428–439
Dahlin MG, Beck OM, Amark PE (2006) Plasma levels of antiepileptic drugs in children on the ketogenic diet. Pediatr Neurol 35(1):6–10
Thorn CF, Aklillu E, Klein TE, Altman RB (2012) PharmGKB summary: very important pharmacogene information for CYP1A2. Pharmacogenet Genomics 22(1):73–77
Zhou SF, Chan E, Zhou ZW, Xue CC, Lai X, Duan W (2009) Insights into the structure, function, and regulation of human cytochrome P450 1A2. Curr Drug Metab 10(7):713–729
Ikeya K, Jaiswal AK, Owens RA, Jones JE, Nebert DW, Kimura S (1989) Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol 3(9):1399–1408
Nordmark A, Lundgren S, Ask B, Granath F, Rane A (2002) The effect of the CYP1A2 *1F mutation on CYP1A2 inducibility in pregnant women. Br J Clin Pharmacol 54(5):504–510
Sachse C, Bhambra U, Smith G, Lightfoot TJ, Barrett JH, Scollay J, Garner RC, Boobis AR, Wolf CR, Gooderham NJ (2003) Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br J Clin Pharmacol 55(1):68–76
Ghotbi R, Christensen M, Roh HK, Ingelman-Sundberg M, Aklillu E, Bertilsson L (2007) Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype-phenotype relationship in Swedes and Koreans. Eur J Clin Pharmacol 63(6):537–546
Chung I, Bresnick E (1997) Identification of positive and negative regulatory elements of the human cytochrome P4501A2 (CYP1A2) gene. Arch Biochem Biophys 338(2):220–226
Wang D, Jiang Z, Shen Z, Wang H, Wang B, Shou W, Zheng H, Chu X, Shi J, Huang W (2011) Functional evaluation of genetic and environmental regulators of p450 mRNA levels. PLoS One 6(10), e24900
Zhou SF, Wang B, Yang LP, Liu JP (2009) Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab Rev 42(2):268–354
Quattrochi LC, Vu T, Tukey RH (1994) The human CYP1A2 gene and induction by 3-methylcholanthrene. A region of DNA that supports AH-receptor binding and promoter-specific induction. J Biol Chem 269(9):6949–6954
Chu-Shore CJ, Thiele EA (2010) New drugs for pediatric epilepsy. Semin Pediatr Neurol 17(4):214–223
Shah J (2004) Criteria influencing the clinical uptake of pharmacogenomic strategies. BMJ 328(7454):1482–1486
Veenstra DL, Higashi MK, Phillips KA (2000) Assessing the cost-effectiveness of pharmacogenomics. AAPS PharmSci 2(3):E29
Delgado Iribarnegaray MF, Santo Bueldga D, Garcia Sanchez MJ, Otero MJ, Falcao AC, Dominguez-Gil A (1997) Carbamazepine population pharmacokinetics in children: mixed-effect models. Ther Drug Monit 19(2):132–139
El Desoky ES, Sabarinath SN, Hamdi MM, Bewernitz M, Derendorf H (2012) Population pharmacokinetics of steady-state carbamazepine in Egyptian epilepsy patients. J Clin Pharm Ther 37(3):352–355
Altafullah I, Talwar D, Loewenson R, Olson K, Lockman LA (1989) Factors influencing serum levels of carbamazepine and carbamazepine-10,11-epoxide in children. Epilepsy Res 4:72–80
Reith DM, Hooper WD, Parke J, Charles B (2001) Population pharmacokinetic modeling of steady state carbamazepine clearance in children, adolescents, and adults. J Pharmacokinet Pharmacodyn 28(1):79–92
Summers B, Summers RS (1989) Carbamazepine clearance in paediatric epilepsy patients. Influence of body mass, dose, sex and co-medication. Clin Pharmacokinet 17(3):208–216
Kong ST, Lim SH, Chan E, Ho PC (2013) Estimation and comparison of carbamazepine population pharmacokinetics using dried blood spot and plasma concentrations from people with epilepsy: the clinical implication. J Clin Pharmacol 54(2):225–233
Carrillo JA, Benitez J (1996) CYP1A2 activity, gender and smoking, as variables influencing the toxicity of caffeine. Br J Clin Pharmacol 41(6):605–608
Tantcheva-Poor I, Zaigler M, Rietbrock S, Fuhr U (1999) Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics 9(2):131–144
Acknowledgments
The study was financially supported by the Faculty of Medical Sciences, University of Kragujevac, Serbia, JP 07/11, and the Ministry of Science and Technology of the Republic of Serbia, grants No. 175007 and 175056.
Contributions of authors’ statement
• Natasa Djordjevic participated in conception and design of the work, as well as in acquisition, analysis, and interpretation of the data, wrote the paper, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Dragana Dragas Milovanovic participated in acquisition, analysis, and interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Marija Radovanovic participated in acquisition, analysis, and interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Ivan Radosavljevic participated in acquisition, analysis, and interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Slobodan Obradovic participated in conception and design of the work, as well as in interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Mihajlo Jakovljevic participated in analysis and interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Dragan Milovanovic participated in acquisition, analysis, and interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Jasmina R. Milovanovic participated in conception and design of the work, as well as in acquisition, analysis, and interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
• Slobodan Jankovic participated in analysis and interpretation of the data, revised the paper critically for important intellectual content, approved the submitted version of the paper, and agrees to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Rights and permissions
About this article
Cite this article
Djordjevic, N., Milovanovic, D.D., Radovanovic, M. et al. CYP1A2 genotype affects carbamazepine pharmacokinetics in children with epilepsy. Eur J Clin Pharmacol 72, 439–445 (2016). https://doi.org/10.1007/s00228-015-2006-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-2006-9