Abstract
Purpose
Systemic exposure to rosuvastatin is approximately double that of Caucasians in Asian subjects. We investigated whether this pattern of increased exposure exists for other statins.
Methods
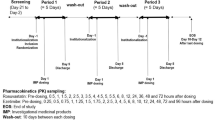
Plasma exposure following single-dose rosuvastatin 20 mg, atorvastatin 40 mg or simvastatin 40 mg was studied in Chinese, Japanese and Caucasian subjects. Plasma concentrations were determined using LC-MS methods. Impact of polymorphisms in SLCO1B1 (T521>C and A388>G) and in ABCG2 (C421>A) on exposure to rosuvastatin, atorvastatin, simvastatin and simvastatin acid was assessed.
Results
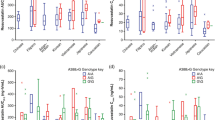
Relative to Caucasians, geometric mean area under the curve from time zero to time of last quantifiable concentration was 86 % (90 % confidence interval (CI), 51–130 %) and 55 % (26–91 %) higher for rosuvastatin in Chinese and Japanese subjects, respectively, 53 % (25–88 %) and 69 % (37–108 %) higher for atorvastatin, 23 % (0–52 %) and 12 % (−0.9–39 %) higher for simvastatin and 28 % (5–56 %) and 34 % (10–64 %) higher for simvastatin acid. Geometric mean maximum drug concentration was also proportionally higher for each statin. Polymorphisms in SLCO1B1 T521>C or ABCG2 C421>A were associated with higher exposure to rosuvastatin, atorvastatin and simvastatin acid (but not simvastatin) within a population, but only the ABCG2 C421>A polymorphism contributed towards between-population exposure differences. In individuals carrying wild-type alleles for both SLCO1B1 and ABCG2, area under the plasma concentration-time curve (AUC) still appeared to be higher for rosuvastatin, atorvastatin and simvastatin acid in Chinese and Japanese subjects compared with Caucasians, respectively.
Conclusion
Increased exposure to statins in Asian subjects versus Caucasians may represent a more general class phenomenon than previously recognized.




Similar content being viewed by others
References
Mabuchi H, Nohara A, Higashikata T, Ueda K, Bujo H, Matsushima T, Ikeda Y, Nii M (2004) Clinical efficacy and safety of rosuvastatin in Japanese patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb 11:152–158
Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, Moore R, Lee C, Chen Y, Schneck D (2005) Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther 78:330–341
Deedwania PC, Gupta M, Stein M, Ycas J, Gold A, IRIS Study Group (2007) Comparison of rosuvastatin versus atorvastatin in South-Asian patients at risk of coronary heart disease (from the IRIS Trial). Am J Cardiol 99:1538–1543
Zhu JR, Tomlinson B, Ro YM, Sim KH, Lee YT, Sriratanasathavorn C (2007) A randomised study comparing the efficacy and safety of rosuvastatin with atorvastatin for achieving lipid goals in clinical practice in Asian patients at high risk of cardiovascular disease (DISCOVERY-Asia study). Curr Med Res Opin 23:3055–3068
Warwick MJ, Dane AL, Raza A, Schneck DW (2000) Single- and multiple-dose pharmacokinetics and safety of the new HMG-CoA reductase inhibitor ZD4522. Atherosclerosis 151:39 (Abstract)
Li Y, Jiang X, Lan K, Zhang R, Li X, Jiang Q (2007) Pharmacokinetic properties of rosuvastatin after single-dose, oral administration in Chinese volunteers: a randomized, open-label, three-way crossover study. Clin Ther 29:2194–2203
Kim K, Birmingham BK, Azumaya CT, Zalikowski J, Chen Y, Schneck D (2008) Increased systemic exposure to rosuvastatin in Asian subjects residing in the United States compared with Caucasian subjects. Clin Pharmacol Ther 83:S14 (Abstract)
Tzeng TB, Schneck DW, Birmingham BK, Mitchell PD, Zhang H, Martin PD, Kung LP (2008) Population pharmacokinetics of rosuvastatin: implications of renal impairment, race, and dyslipidaemia. Curr Med Res Opin 24:2575–2585
Martin PD, Warwick MJ, Dane AL, Brindley C, Short T (2003) Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther 25:2553–2563
Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, Lenz E (2003) Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther 25:2822–2835
Choi JH, Lee MG, Cho JY, Lee JE, Kim KH, Park K (2008) Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin Pharmacol Ther 83:251–257
Kitamura S, Maeda K, Wang Y, Sugiyama Y (2008) Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos 36:2014–2023
Tirona RG, Leake BF, Merino G, Kim RB (2001) Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 276:35669–35675
Huang L, Wang Y, Grimm S (2006) ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos 34:738–742
Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M (2009) ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther 86:197–203
Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Zalikowski J, Chen Y, Kim K, Ambrose HJ (2015) Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol doi: 10.1007/s00228-014-1800-0
Tirona RG (2005) Ethnic differences in statin disposition. Clin Pharmacol Ther 78:311–316
Elsby R, Hilgendorf C, Fenner K (2012) Understanding the critical disposition pathways of statins to assess drug-drug interaction risk during drug development: it’s not just about OATP1B1. Clin Pharmacol Ther 92:584–598
Gandelman K, Fung GL, Messig M, Laskey R (2012) Systemic exposure to atorvastatin between Asian and Caucasian subjects: a combined analysis of 22 studies. Am J Ther 19:164–173
Zhang W, Yu BN, He YJ, Fan L, Li Q, Liu ZQ, Wang A, Liu YL, Tan ZR, Fen-Jiang, Huang YF, Zhou HH (2006) Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta 373:99–103
Lee HK, Hu M, Lui SS, Ho CS, Wong CK, Tomlinson B (2013) Effects of polymorphisms in ABCG2, SLCO1B1, SLC10A1 and CYP2C9/19 on plasma concentrations of rosuvastatin and lipid response in Chinese patients. Pharmacogenomics 14:1283–1294
Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M (2007) Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther 82:726–733
Lau YY, Huang Y, Frassetto L, Benet LZ (2007) Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther 81:194–204
Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M (2006) SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics 16:873–879
Allred AJ, Bowen CJ, Park JW, Peng B, Williams DD, Wire MB, Lee E (2011) Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers. Br J Clin Pharmacol 72:321–329
Tomita Y, Maeda K, Sugiyama Y (2013) Ethnic variability in the plasma exposures of OATP1B1 substrates such as HMG-CoA reductase inhibitors: a kinetic consideration of its mechanism. Clin Pharmacol Ther 94:37–51
Acknowledgments
We acknowledge Debra Ennis for her contributions as study delivery director. We thank Kerry Knight and Valerie Moss from Prime Medica Ltd and Maren White from White Quill Ltd who provided medical writing and editorial support funded by AstraZeneca.
Responsibility for opinions, conclusions and interpretation of data lies with the authors.
Sources of financial support
This study was funded by AstraZeneca.
Conflict of interest
B.K. Birmingham, C.T. Azumaya, C. Wei, H.J Ambrose, R. Elsby and S. R. Bujac are all employees of AstraZeneca. R. Mosqueda-Garcia and Y. Chen are former employees of AstraZeneca.
C.T. Azumaya, C. Wei, R. Mosqueda-Garcia, Helen J. Ambrose, R. Elsby and S. R. Bujac hold stock in AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 3209 kb)
Rights and permissions
About this article
Cite this article
Birmingham, B.K., Bujac, S.R., Elsby, R. et al. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect?. Eur J Clin Pharmacol 71, 341–355 (2015). https://doi.org/10.1007/s00228-014-1801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1801-z