Abstract
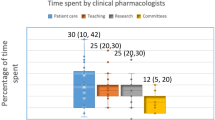
Clinical pharmacology in Russia has long history and is currently active, but rather unrecognized internationally. It is governmentally approved as a teaching/scientific specialty since 1983 and as a medical specialty since 1997. Courses of clinical pharmacology are included in the undergraduate curricula in the 5th and/or 6th year of education at all medical schools in the Russian Federation. Postgraduate education includes initial specialization in internal medicine with further residency in clinical pharmacology. Governmental legislation recommends that every healthcare institution has either a department or a single position of clinical pharmacologist. Major routine duties include information about and monitoring of medication use, consultations in difficult clinical situations, pharmacogenetic counseling, therapeutic drug monitoring, pharmacovigilance, and participation in drug and therapeutics (formulary) committees. There are official experts in clinical pharmacology in Russia responsible for coordinating relevant legislative issues. The chief expert clinical pharmacologist represents the discipline directly at the Ministry of Health. Research in clinical pharmacology in Russia is extensive and variable, but only some of it is published internationally. Russia is a participant of international societies of clinical pharmacology and therapeutics and collaboration is actively ongoing. There are still certain problems related to the development of the discipline in Russia—some healthcare institutions do not see the need for clinical pharmacology. However, the number of clinical pharmacologists in Russia is increasing as well as their role in physicians’ education, national healthcare, and research.
Similar content being viewed by others
References
WHO, CIOMS, IUPHAR (2012) Clinical pharmacology in healthcare, teaching and research. World Health Organization, IUPHAR and CIOMS. 1–75
Birkett D, Brøsen K, Cascorbi I et al (2010) Clinical pharmacology in research, teaching and health care: considerations by IUPHAR, the International Union of Basic and Clinical Pharmacology. Basic Clin Pharmacol Toxicol 107:531–559. doi:10.1111/j.1742-7843.2010.00602.x
Votchal BE (1963) Assays on clinical pharmacology. Gosudarstvennoe Izdatelstvo Medicinskoy Literatury Moscow, p 416
Dollery CT (1966) Clinical pharmacology. Lancet 287:359–360. doi:10.1016/S0140-6736(66)91337-7
Sjöqvist F (2014) Development of clinical pharmacology as a medical speciality in Europe—the roles of WHO, IUPHAR and EACPT. Basic Clin Pharmacol Toxicol. doi:10.1111/bcpt.12278
WHO Study Group on Clinical Pharmacology (1970) Clinical pharmacology scope, organization, training: report of a WHO study group [meeting held in Geneva from 8 to 12 December 1969]. http://apps.who.int//iris/handle/10665/40774. Accessed 17 November 2014
Orme M, Frolich J, Vrhovac B (2002) Towards a core curriculum in clinical pharmacology for undergraduate medical students in Europe. Eur J Clin Pharmacol 58:635–640. doi:10.1007/s00228-002-0531-9
Kukes V (2013) Clinical pharmacology, 4th ed. GEOTAR-media, Moscow, p 1052
Belousov Y, Kukes V, VL, Petrov V (2014) Clinical pharmacology. National guide. GEOTAR-media, Moscow, p 976
Petrov V (2014) Clinical pharmacology and pharmacotherapy in real clinical practice. GEOTAR-media, Moscow, p 880
Gilman AG (2006) Clinical pharmacology by Goodman & Gilman in 4 books. Praktika, Moscow
Laurence D, Bennet P, Brown M (2002) Clinical pharmacology, 2nd ed. Medicina, Moscow, p 996
Katzung BG (2007) Basic and clinical pharmacology, 2nd ed. Binom Dialekt, Moscow, p 648
Sychev DA, Antonov IM, Ignat’ev IV et al (2010) Anticoagulant action and safety of warfarin dosing based on pharmacogenetic testing: results of the first Russian prospective pilot study. Kardiologiia 50:42–46
Pchelina SN, Sirotkina OV, Taraskina AE et al (2005) The frequency of cytochrome P450 2C9 genetic variants in the Russian population and their associations with individual sensitivity to warfarin therapy. Thromb Res 115:199–203. doi:10.1016/j.thromres.2004.08.020
Zhestovskaja AS, Kukes VG, Sychev DA (2013) Personalized medicine: myth or reality? The position of Russian clinical pharmacologists. EPMA J 4:13. doi:10.1186/1878-5085-4-13
Gerasimova KV, Sychev DA, Avksentyeva MV, Kukes VG (2010) Awareness about pharmacogenetic testing and its availability among healthcare professionals in Russia. Klinich Farmakol Farmakoecon 3:12–18
World Health Assembly 20 (1967) WHO pilot research project for international monitoring of adverse reaction to drugs. http://apps.who.int/iris/handle/10665/89523 Accessed 17 November 2014
Orme M, Sjöqvist F (2013) Clinical pharmacology in European health care—outcome of a questionnaire study in 31 countries. Eur J Clin Pharmacol 69:1635–1639. doi:10.1007/s00228-013-1519-3
Acknowledgments
We would like to acknowledge Professor Folke Sjoqvist for the conceptualization of this review and for the advice on the content and editing of the manuscript and Professor Michael Orme for the thorough reading and linguistic editing of the text.
Author contributions
Dr. Zagorodnikova drafted the initial manuscript and revised it. Dr. Burbello retrieved historical data presented in the text, supervised, and revised the manuscript. Dr. Sychev collected and provided data on research in clinical pharmacology and revised the manuscript. Dr. Frolov provided information on professional associations and collaboration as well as official statistical figures related to the current state of clinical pharmacology. Dr. Kukes co-supervised and revised all sections of the manuscript, mainly in history and education parts. Dr. Petrov co-supervised the review and contributed to its concept.
Ethical considerations
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Professional education for clinical pharmacologists in Russia (DOC 26 kb)
Supplementary Figure 2
Administrative representation of clinical pharmacology in Russia (DOC 28 kb)
Supplementary Figure 3
Regional distribution of clinical pharmacologists in Russia (number per 1,000 inhabitants) (DOC 57 kb)
Rights and permissions
About this article
Cite this article
Zagorodnikova (Goryachkina), K., Burbello, A., Sychev, D. et al. Clinical pharmacology in Russia—historical development and current state. Eur J Clin Pharmacol 71, 159–163 (2015). https://doi.org/10.1007/s00228-014-1787-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1787-6