Abstract
Purpose
Dolutegravir (DTG), an unboosted HIV integrase inhibitor (INI), is metabolized by UGT1A1 and to a minor extent by CYP3A. Renal elimination of unchanged DTG is very low (< 1 %). As renal impairment may affect pharmacokinetics (PK), even for drugs primarily metabolized or secreted in bile, this study investigated the effect of renal impairment on the PK of DTG.
Methods
This was an open-label, single-dose study of oral DTG 50 mg administered to subjects with severe renal impairment (creatinine clearance [CLcr] <30 mL/min; not on dialysis) and to healthy controls (CLcr >90 mL/min) matched for gender, age and body mass index (8 subjects per group). Serial PK samples were collected up to 72 h post-dose for determination of DTG and DTG-glucuronide (DTG-Gluc) concentrations in plasma. DTG unbound fraction in plasma was determined at 3 and 24 h. PK parameters were determined by non-compartmental methods and compared between groups by analysis of covariance.
Results
DTG was well tolerated with a low incidence of Grade 1 adverse events. DTG PK parameters showed significant overlap between groups. DTG mean exposure was lower in subjects with severe renal impairment compared to healthy, matched subjects: AUC(0-∞) and Cmax were 40 % and 23 % lower, while mean DTG-Gluc was increased. Renal impairment did not affect DTG fraction unbound in plasma.
Conclusions
The modest reductions in mean PK exposures for DTG and increases for DTG-Gluc in the severe renal impairment group are not considered clinically significant. DTG does not require dose adjustment in patients with renal impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dolutegravir (DTG) is an investigational integrase inhibitor (INI) for treatment of HIV infection which has demonstrated safety and efficacy in Phase 3 trials [1–3]. Its unique resistance profile supports its antiviral activity in patients who have previously failed treatment with the INI raltegravir [4].
The pharmacokinetic (PK) profile of DTG is characterized by a long half-life supporting once-daily dosing in INI-naïve subjects, no requirement for boosting with ritonavir, and a well-characterized PK/pharmacodynamic relationship demonstrated in short-term monotherapy [5, 6]. DTG is primarily metabolized via glucuronidation by UGT1A1 in the liver, and to a minor extent by CYP3A. It is greater than 99 % bound to plasma proteins [7]. DTG is the predominant circulating compound in plasma (>97 % of drug-related components) and renal elimination of unchanged drug is extremely low (<1 % of the dose) [8]. Approximately 53 % of a dose is recovered as DTG in feces and approximately 31 % is excreted in the urine primarily as DTG-glucuronide (DTG-Gluc) and other minor metabolites [8].
Severe renal impairment can impact the PK of drugs which are primarily metabolized or secreted in bile [9]. Current regulatory guidance recommends evaluation of drug PK in subjects with renal impairment, even for drugs that are primarily metabolized [10]. Accordingly, this study investigated if renal impairment significantly affects the PK of DTG.
Methods
Study design and participants
This was a single-center, Phase I, single-dose, open-label study in human subjects with severely impaired renal function who are not on renal replacement therapy, in comparison to a matched group of healthy subjects with normal renal function. Severe renal impairment was defined as a creatinine clearance (CLcr) <30 mL/min based on 24-hour urine creatinine clearance within 30 days of the treatment period. Healthy subjects were required to have CLcr >90 mL/min based on 24-hour urine creatinine clearance within 30 days of the treatment period and were matched to severe renal impaired subjects for gender, age (±5 years) and body mass index (±25 %). As race/ethnicity has not been shown to affect DTG pharmacokinetics, this was not used as a factor for matching [11]. A sample size of eight subjects per group was chosen based on regulatory guidance for studies in renal impairment [10].
HIV-seronegative male and female participants between 18 to 70 years of age and with a body weight ≥50 kg and BMI in the range of 19 to 38 kg/m2 were eligible for enrollment. Subjects with renal impairment were enrolled if they met the CLcr inclusion criteria and had laboratory test results that were considered clinically stable by the principal investigator (PI). Renal impairment subjects were excluded for recent hepatitis B infection, a positive HIV antibody and pre-existing conditions (other than renal impairment) that might interfere with normal gastrointestinal anatomy or motility and/or those that could interfere with absorption, metabolism, or excretion of the study drug. All subjects could not have consumed red wine, Seville oranges or grapefruit products within 7 days prior to the dose of study medication until the collection of the last PK sample, but renally impaired subjects were permitted to use concomitant medications that were considered medically necessary by the PI and did not have the potential to affect DTG exposure, as determined by effects on DTG metabolic pathways. Antacids, vitamins, and supplements were held on the day of dosing.
Healthy participants were determined to be eligible for inclusion based on physical exam, medical history, and laboratory evaluation. They could not receive any prescription or nonprescription drugs or consume red wine, Seville oranges or grapefruit products within 7 days prior to the dose of study medication until the collection of the last PK sample. Furthermore, exclusion criteria for healthy participants included a positive pre-study drug screen for drugs of abuse.
Subjects received a single 50 mg tablet of DTG in the morning in a fasted state and were housed in the study center until collection of the last PK sample. Blood samples for determination of total DTG and DTG-Gluc plasma concentrations were collected pre-dose and at 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, and 72 h post-dose. Additional plasma samples were collected at 3 and 24 h post-dose for measurement of unbound plasma concentrations of DTG. Safety was assessed throughout the study by clinical and laboratory evaluations and at follow-up 7 to 10 days after administration of the DTG dose.
Written informed consent was obtained from all participants prior to conduct of any study-specific procedures and the protocol was approved by the IRB (Western Institutional Review Boards, Olympia, WA).
Bioanalytical methods
DTG (MW = 419.38) and DTG-Gluc (MW = 594.51) concentrations in plasma were determined by validated, high performance liquid chromatographic/tandem mass spectrometric methods following extraction with acetonitrile. Determination of unbound DTG concentration was conducted by equilibrium dialysis. Details of the analytical method and assay performance are provided in the supplementary document.
Pharmacokinetic analysis
Analysis of the DTG and DTG-Gluc concentration-time data was performed by noncompartmental PK methods (WinNonlin® Professional Edition 5.2; Pharsight Corporation, Mountain View, CA). Plasma DTG PK parameters calculated included area under the plasma concentration–time curve from time zero to infinity (AUC(0-∞)), area under the plasma concentration–time curve from time zero to the last quantifiable time point [AUC(0-t)], maximum observed plasma concentration (Cmax), time to Cmax (tmax), concentration at 24 h after dosing (C24), apparent oral clearance (CL/F), apparent volume of distribution (Vz/F), and terminal elimination phase half-life (t1/2). The same PK parameters were determined for DTG-Gluc except for CL/F, Vz/F and C24. Additionally, molar ratios of DTG-Gluc/DTG were determined for AUC(0-t) and Cmax, as was the DTG-Gluc/DTG t1/2 ratio.
Unbound fraction (fu) was calculated using the total and unbound plasma concentration of DTG data generated at 3 and 24 h post-dose for both healthy and renal impairment subjects using the following formula: fu = C(unbound)/C(total), where C(unbound) and C(total) are the unbound and total concentration of DTG in plasma, respectively.
Statistical analysis
Statistical analysis was performed on the log-transformed PK parameters, except tmax and tlag. Analysis of covariance (ANCOVA) was performed using the SAS system (Version 9.1; SAS Institute Inc, Cary, NC) with gender as a fixed effect and with age and BMI as continuous covariates. For each log-transformed PK parameter, the point estimate and its associated 90 % confidence interval (CI) was constructed for the difference between subjects with renal impairment (test) and matched healthy controls (reference). The difference in PK parameter and its 90 % CI was then exponentiated to obtain the ratio of geometric least squares means and its 90 % CI on the original scale. For DTG-Gluc/DTG ratios, Hodges–Lehmann estimates of difference and 90 % CI were determined. Pearson correlation coefficients between creatinine clearance and DTG parameters and creatinine clearance and DTG-Gluc PK parameters were provided.
Results
Study population
Sixteen subjects (8 renal impairment; 8 healthy) were enrolled and completed all study assessments. Summary demographics are provided in Table 1. Groups were similar in age, gender and BMI given the matching criteria. There were 5 African-Americans and 3 Caucasians in the renal impairment group and 2 African-Americans and 6 Caucasians in the healthy subject group. Concomitant medications in the renal impairment group were most commonly administered for treatment of hypertension, diabetes, and lipid disorders.
Pharmacokinetics
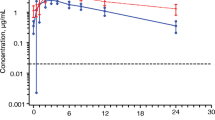
Mean concentration-time profiles of DTG and DTG-Gluc in subjects with and without renal impairment are shown in Fig. 1. Summary DTG pharmacokinetic parameter estimates are provided in Table 2. Subjects with severe renal impairment had an average 40 % lower plasma DTG AUC(0-∞) and 23 % lower Cmax than subjects with normal renal function. This was accompanied by a mean elimination half-life that was shorter by 18 % in the subjects with renal impairment compared to controls (12.7 vs 15.4 h, respectively). There was considerable overlap in the plasma DTG PK parameters and in the direction of the change between renally impaired and healthy subjects. When compared to their matched controls, 6/8 healthy subjects had a higher AUC while 2/8 renally impaired subjects had a higher AUC. Values for AUC(0-∞) ranged from 13.6 to 46.7 μg*h/ml in renal impairment subjects and from 14.1 to 60.9 μg*h/ml in matched healthy subjects. For Cmax, 4/8 healthy subjects had a higher value while 3/8 renally-impaired subjects had a higher value and one matched pair demonstrated similar a Cmax (1.8 vs 1.9 μg/mL). Cmax ranged from 0.79 to 2.1 μg/ml in renal impairment subjects and from 0.82 to 3.2 μg/ml in healthy subjects. Variability in DTG PK parameters was similar between the two groups (Table 2). No significant correlation was observed between creatinine clearance and DTG PK parameters (p > 0.1).
The unbound fraction (fu) was similar between the severe renal impairment and normal renal function groups, but the GLS mean unbound plasma DTG concentrations were 14 % lower at the 3-hour time point and 49 % lower at the 24-hour time point in the renal impaired subjects (Table 2). Variability in DTG unbound fraction was higher in subjects with severe renal impairment. There was no apparent correlation between DTG unbound fraction and DTG total concentration in plasma, suggesting that protein binding is independent of DTG concentration over the range of concentrations observed in this study.
Plasma DTG-Gluc AUC(0-∞) and Cmax in the subjects with severe renal impairment were approximately fourfold and threefold higher than those in the healthy subjects (Table 3). The median molar DTG-Gluc-to-DTG AUC ratio increased from 0.011 in healthy subjects to 0.053 in subjects with severe renal impairment, and the median molar DTG-Gluc-to-DTG Cmax ratio increased from 0.015 in healthy subjects to 0.045 in subjects with severe renal impairment. The molar DTG-Gluc-to-DTG ratio of AUC and Cmax expressed as a percentage was 1–5.5 %, indicating that DTG is the predominant species in plasma, even in renal impairment. AUC(0-t) and Cmax ratios of DTG-Gluc to DTG were higher in subjects with severe renal impairment than healthy matched subjects. The t1/2 ratio of DTG-Gluc to DTG is close to 1 in both groups of subjects, indicating that the terminal t1/2 of DTG-Gluc is similar to that of DTG, and that the formation-rate-limited kinetics for DTG-Gluc was not altered by severe renal impairment. There was a statistically significant negative correlation between creatinine clearance and DTG-Gluc PK parameters including AUC(0-t), AUC(0-∞) and Cmax (p < 0.05, correlation coefficients range -0.54 to -0.60), but not for the t1/2 of DTG-Gluc (p > 0.05, correlation coefficient = −0.07).
Safety
DTG was well tolerated in both treatment groups. No deaths or serious adverse events occurred during this study. No subjects were withdrawn from the study due to adverse events (AEs) and all AEs were mild (Grade 1) in intensity. One subject with severe renal impairment experienced an AE (dizziness) considered by the investigator to be related to the study drug. The AE occurred approximately 15 min after dosing, resolved within 20 min and no action was taken. There were no drug-related AEs in the healthy subject group.
Discussion
DTG is primarily eliminated through metabolism, and renal elimination of unchanged drug represents less than 1 % of the total dose administered. A study using a radio-labeled drug demonstrated that approximately 30 % of the total oral dose is excreted in the urine, the majority of which is represented by a glucuronidated metabolite with a small percentage of oxidative and other minor metabolites [8]. Approximately 65 % of the total oral dose is recovered in the feces, represented mainly by parent drug (53 % of total dose) [8]. Given this metabolism and excretion profile, renal impairment would be expected to have a minor effect on DTG PK.
The results of this study showed that the mean total DTG plasma Cmax and AUC(0-∞) were 23–40 % lower in subjects with severe renal impairment compared to those in matched, healthy subjects. However, there was considerable overlap in DTG PK parameters between the groups. Median tmax values were similar between the two subject populations, as were the values for fraction unbound in plasma at 3 and 24 h post-dose. Mean half-life was modestly shorter in the renally impaired (12.7 h) group compared to the healthy controls (15.4 h). There was no correlation between creatinine clearance and DTG exposure. It has been well described that renal impairment can lead to a number of changes that may affect the pharmacokinetics of non-renally cleared drugs, including decreased hepatic and intestinal CYP activity, decreased intestinal Pgp and MRP2 activity, and decreased or minimal impact on hepatic OATP activity [12–15]. However, these previously described effects on drug-metabolizing enzymes and transporters would result in a higher exposure in the renal impairment group, in contrast to the overall lower exposure seen in this study.
Previous studies have demonstrated reduced exposure of acidic drugs such as phenytoin in subjects with severe renal impairment with an accompanied higher free fraction in plasma and no significant change in the unbound exposure [16]. A possible explanation for the findings in the current study is a reduction in absorption. Patients with severe renal impairment may have alterations in gastrointestinal transit time or bacterial overgrowth in the gastrointestinal tract that may affect drug absorption [17, 18]. There is evidence that DTG undergoes enterohepatic recirculation [8] and gastrointestinal changes in these subjects may impair this mechanism and contribute to lower exposure in some patients.
The calculated free fraction was similar between renally impaired and normal subjects, which may be explained by the similar albumin levels between the two groups with no change in the association constant. Therefore, the reduction in unbound DTG plasma concentration is driven by the reduction in total DTG plasma concentration, not free fraction. The free fraction (fu) observed in the healthy matched subjects in this study is higher than that observed in a previous study in healthy subjects [19]. The reason for this difference is unclear, but a potential contributor is the slightly lower albumin concentration noted for the healthy subjects in this study resulting in higher free fractions.
Although DTG-Gluc concentrations are much lower than the total DTG concentrations, the plasma exposure of DTG-Gluc in renally impaired subjects was 3 times (for Cmax) and 4 times (for AUC) higher than in normal subjects. However, the half-life of DTG glucuronide was similar between the two groups and similar to the DTG half-life, indicating formation rate-limited kinetics for the glucuronide metabolite was not altered by severe renal impairment. This suggests that the glucuronide would not be expected to accumulate differentially in renally impaired subjects compared to those with normal function on repeat dosing. From a clinical standpoint, the DTG-Gluc metabolite has no antiviral activity and would not contribute to efficacy in the HIV-infected population. Glucuronidation of DTG disrupts the key metal binding motif of the compound and completely abrogates any antiviral activity resulting from the active site binding to the integrase enzyme. The increased DTG-Gluc concentrations in the renally-impaired population would also not be expected to be a safety concern as the glucuronide is still <10 % of the exposure of parent DTG based on molar ratios. As such, the glucuronide would not be considered for safety assessment studies according to regulatory guidance on safety testing for metabolites [20]. In addition, the glucuronide is a Phase II metabolite; these metabolites are considered generally more water soluble and less pharmacologically active, and do not require testing [20].
The moderate effect of severe renal impairment in reducing DTG Cmax and AUC by approximately 23–40 % is not considered clinically significant. The reduction is less than the 75 % reduction in DTG exposure between the 50 mg and 10 mg once-daily doses observed in a dose-ranging study of DTG in treatment-naïve subjects (SPRING-1) [21]. This trial demonstrated that all DTG doses (10, 25, and 50 mg) combined with a 2 drug nucleoside reverse transcriptase inhibitor backbone were equally efficacious and showed comparable safety. This suggests that the decrease in DTG exposure observed in the current study (approximately 40 %) would not be clinically relevant in contemporary HIV treatment regimens. Further support for this conclusion is provided by results from co-administration of DTG with darunavir/ritonavir (DRV/r). In a Phase 1 study, DRV/r reduced DTG trough concentration by 38 % [22]. However, in the Phase 3 SAILING study of treatment-experienced but INI-naive subjects, those subjects taking DTG and DRV/r (accounting for ∼40 % subjects enrolled) showed equivalent virologic response to the remainder of the DTG-treated population, despite lower exposure [3]. Therefore, it is unlikely that the decrease in exposure seen in this study of subjects with severe renal impairment would have a deleterious effect on virologic response.
This study indicates that no dose adjustment is needed in INI-naïve subjects with severe renal impairment (CLcr of < 30 mL/min, not on renal replacement therapy) and therefore also extends to those with mild and moderate impairment. However, caution should be given to subjects with severe renal impairment and who require higher exposures, such as those with resistance to raltegravir. Furthermore, since metabolic inducers such as efavirenz and tipranavir/ritonavir can decrease DTG concentrations, alternative antiretroviral regimens without such drugs should be considered in subjects with severe renal impairment receiving DTG. Dose recommendations for DTG in end-stage renal disease patients receiving any form of renal replacement therapy cannot be given at the current time, although it is unlikely that such therapy would have a significant effect on DTG PK given the very high protein binding of >99 %.
References
Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, Bloch M, Podzamczer D, Pokrovsky V, Pulido F, Almond S, Margolis D, Brennan C, Min S (2013) Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 381:735–743. doi:10.1016/S0140-6736(12)61853-4
G Nichols, A Mills, R Grossberg, et al. (2012) Antiviral activity of dolutegravir in subjects with failure on an integrase inhibitor-based regimen: week 24 phase 3 results from VIKING-3. 11th International Congress on Drug Therapy in HIV Infection (HIV11). Glasgow, November 11–15, 2012. Abstract O232.
Cahn P, Posniak AL, Mingrone H, et al (2013) Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 382:700–708
Underwood MR, Johns BA, Sato A, Martin JN, Deeks SG, Fujiwara T (2012) The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. J Acquir Immune Defic Syndr 61(3):297–301
Min S, Song I, Borland J et al (2010) Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother 54:254–258
Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, Stroder R, Chen S, Underwood M, Fujiwara T, Piscitelli S, Lalezari J (2011) Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 25(14):1737–45
Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, Webster LO, Harmon KA, Clarke JD, Polli JW (2013) In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos 41:353–61
Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S, Lou Y, Min S, Goljer I, Culp A, Piscitelli SC, Savina PM (2013) Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor, dolutegravir, in humans. Antimicrob Agents Chemother. doi:10.1128/AAC.00292-13, ePub ahead of print; 13 May 2013
Dreisbach AW, Lertora JJL (2003) The effect of chronic renal failure on hepatic drug metabolism and drug disposition. Semin Dial 16(1):45–50
Food and Drug Administration. (2010) Guidance for industry: Pharmacokinetics in patients with impaired renal function — study design, data analysis, and impact on dosing and labeling. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf. Accessed 27 May 2013.
TIVICAY (dolutegravir) prescribing information. ViiV Healthcare, Research Triangle Park, NC, August 2013.
Pichette V, Leblond F (2003) Drug metabolism in chronic renal failure. Current Drug Metabolism 4:91–103
Sun H, Frassetto L, Benet L (2006) Effects of renal failure on drug transport and metabolism. Pharmacology & Therapeutics 109:1–11
Naud J, Michaud J, Boisvert C, Desbiens K, Leblond F, Mitchell A, Jones C, Bonnardeaux A, Pichette V (2007) Down-regulation of intestinal drug transporters in chronic renal failure in rats. J Pharmacol Exp Ther 320(3):978–985
Naud J, Michaud J, Leblond F, Lefrancois S, Bonnardeaux A, Pichette V (2008) Effects of chronic renal failure on liver drug transporters. Drug Met Dispos 36:124–128
Liponi DF, Winter ME, Tozer TN (1984) Renal function and therapeutic concentrations of phenytoin. Neurology 34(3):395–7
Lefebvre HP, Ferré J-P, Watson DJ A, Brown CA, Serthelon J-P, Laroute V, Concordet D, Toutain P-L (2001) Small bowel motility and colonic transit are altered in dogs with moderate renal failure. Am J Physiol Regul Integr Comp Physiol 281:R230–R238
Strida H, Simrén M, Stotzer P-O, Ringström G, Abrahamsson H, Björnsson ES (2003) Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion 67:129–137
Song I, Borland J, Savina P, Chen S, Patel P, Wajima T, Peppercorn A, Piscitelli S. (2012) Pharmacokinetics of dolutegravir in subjects with moderate hepatic impairment. 19th Conference on Retroviruses and Opportunistic Infections. March 5–8, 2012, Seattle, WA, Abstract 608.
Food and Drug Administration. (2008) Guidance for industry: Safety testing of drug metabolites. February 2008. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM079266.pdf Accessed 27 May 2013.
van Lunzen J, Maggiolo F, Arribas JR et al (2012) Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 12:111–118
Song I, Min SS, Borland J, Lou Y, Chen S, Patel P, Ishibashi T, Piscitelli SC (2011) The effect of lopinavir/ritonavir and darunavir/ritonavir on the HIV integrase inhibitor S/GSK1349572 in healthy participants. J Clin Pharmacol 51(2):237–42
Financial disclosure
Funding for this study was provided by ViiV Healthcare LLC. Stephen Weller, Julie Borland, Shuguang Chen, Mark Johnson, Paul M. Savina, Brian Wynne, Amanda F. Peppercorn, and Stephen C. Piscitelli are employees of GlaxoSmithKline. Toshihiro Wajima is an employee of Shionogi & Co., LTD.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov identifier: NCT01353716
These data were presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, September 10–13, 2013, Denver, CO, USA
Electronic supplemenatry materials
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Weller, S., Borland, J., Chen, S. et al. Pharmacokinetics of dolutegravir in HIV-seronegative subjects with severe renal impairment. Eur J Clin Pharmacol 70, 29–35 (2014). https://doi.org/10.1007/s00228-013-1590-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1590-9