Abstract
Purpose
Information about dosing adjustments in patients with chronic kidney disease is important to avoid toxicity for several medicines. The aim of our study was to assess the clinical relevance of the instructions for dose adjustment in patients with renal impairment provided in the Summaries of Product Characteristics (SmPCs) approved by the European Medicines Agency (EMA).
Methods
SmPCs available on the EMA website on April 2011 were retrieved, and information on the elimination route and instructions for use in renal impairment was analysed independently by two of the authors. SmPCs were classified as containing ‘explicit’ or ‘poor’ information based on whether they presented (or not) instructions for use of the medicine in renal impairment. Information was considered ‘relevant’ if SmPCs provided clear instructions for dose adjustment.
Results
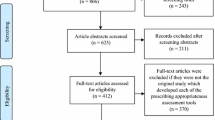
Of the 356 SmPCs analysed, 13.8 and 37.4 % were classified as providing poor information and explicit but not relevant information, respectively. Only 48.8 % SmPCs provided both explicit and relevant information on medicine use in renal impairment. No difference was found in the average time since last update among SmPCs classified as containing explicit or poor information, as well as those classified as containing relevant or not relevant information. Also, no association was found between the clinical relevance of the information and whether or not the medication was an orphan drug, and 80 % SmPCs did not provide information on the use of the medicine in patients undergoing haemodialysis.
Conclusions
Based on our analysis, current versions of SmPCs are characterised by several information deficits and by containing recommendations that are not relevant to clinical practice in terms of dose adjustment in renal impairment. These shortcomings might limit their usefulness for healthcare professionals and integration into clinical decision-making support systems.
Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279(15):1200–1205
Kaushal R, Shojania KG, Bates DW (2003) Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med 163(12):1409–1416
Saxena K, Lung BR, Becker JR (2011) Improving patient safety by modifying provider ordering behavior using alerts (CDSS) in CPOE system. AMIA Annu Symp Proc 2011:1207–1216
Kuperman GJ, Teich JM, Gandhi TK, Bates DW (2001) Patient safety and computerized medication ordering at Brigham and Women’s Hospital. Jt Comm J Qual Improv 27(10):509–521
Kaushal R, Bates DW (2001) Chapter 6. Computerized physician order entry (CPOE) with clinical decision support systems (CDSSs). In: Shojania KG, Duncan BW, McDonald KM, Wachter RM (eds) Making health care safer: a critical analysis of patient safety practices. AHRQ, Rockville
Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PR, Noskin GA (2004) The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med 164(7):785–792
Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander Vliet M, Seger DL (1998) Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. Jama 280(15):1311–1316
Maxwell S, Eichler HG, Bucsics A, Haefeli WE, Gustafsson LL (2012) e-SPC—delivering drug information in the 21st century: developing new approaches to deliver drug information to prescribers. Br J Clin Pharmacol 73(1):12–15
European Commission (2009) Notice to Applicants. A guideline on Summary of Product Characteristics (SmPC). Available at:http://ec.europa.eu/health/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf Accessed 25-May-2011
European Medicines Agency (2013) How to prepare and review a Summary of Product Characteristics. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000357.jsp&mid=WC0b01ac05806361e1. Accessed 04-May-2013
San Miguel MT, Martinez JA, Vargas E (2005) Food-drug interactions in the summary of product characteristics of proprietary medicinal products. Eur J Clin Pharmacol 61(2):77–83
Fusier I, Tollier C, Husson MC (2005) Infovigilance: reporting errors in official drug information sources. Pharm World Sci 27(3):166–169
Ferner RE, Coleman J, Pirmohamed M, Constable SA, Rouse A (2005) The quality of information on monitoring for haematological adverse drug reactions. Br J Clin Pharmacol 60(4):448–451
Pestotnik SL, Classen DC, Evans RS, Stevens LE, Burke JP (1993) Prospective surveillance of imipenem/cilastatin use and associated seizures using a hospital information system. Ann Pharmacother 27(4):497–501
Jick H (1977) Adverse drug effects in relation to renal function. Am J Med 62(4):514–517
Martin-Facklam M, Rengelshausen J, Tayrouz Y, Ketabi-Kiyanvash N, Lindenmaier H, Schneider V, Bergk V, Haefeli WE (2005) Dose individualisation in patients with renal insufficiency: does drug labelling support optimal management? Eur J Clin Pharmacol 60(11):807–811
Pillans PI, Landsberg PG, Fleming AM, Fanning M, Sturtevant JM (2003) Evaluation of dosage adjustment in patients with renal impairment. Intern Med J 33(1–2):10–13
Bertsche T, Fleischer M, Pfaff J, Encke J, Czock D, Haefeli WE (2009) Pro-active provision of drug information as a technique to address overdosing in intensive-care patients with renal insufficiency. Eur J Clin Pharmacol
Roberts GW, Farmer CJ, Cheney PC, Govis SM, Belcher TW, Walsh SA, Adams RJ (2010) Clinical decision support implemented with academic detailing improves prescribing of key renally cleared drugs in the hospital setting. J Am Med Inform Assoc 17(3):308–312
Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, Lee R, Mekala A, Song J, Komaroff AL, Bates DW (2001) Guided medication dosing for inpatients with renal insufficiency. JAMA 286(22):2839–2844
Wang HY, Lu CL, Wu MP, Huang MH, Huang YB (2012) Effectiveness of an integrated CPOE decision supporting system with clinical pharmacist monitoring practice in preventing antibiotic dosing errors. Int J Clin Pharmacol Ther 50(June):375–382
Coleman JJ, Nwulu U, Ferner RE (2012) Decision support for sensible dosing in electronic prescribing systems. J Clin Pharm Ther 37(4):415–419
Dowling TC, Wang ES, Ferrucci L, Sorkin JD (2013) Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore longitudinal study on aging: impact on renal drug dosing. Pharmacotherapy. doi: 10.1002/phar.1282
Sim J, Wright CC (2005) The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 85(3):257–268
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Rajpal A, Reidenberg MM (2003) Drug labeling should be kept current. Clin Pharmacol Ther 73(1):4–6
European Medicines Agency (2007) Orphan drugs and rare diseases at a glance. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2010/01/WC500069805.pdf. Accessed 16 Apr 2013
Kesselheim AS, Myers JA, Avorn J (2011) Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. Jama 305(22):2320–2326
Joppi R, Bertele V, Garattini S (2013) Orphan drugs, orphan diseases. The first decade of orphan drug legislation in the EU Eur J Clin Pharmacol 69(4):1009–1024
European Medicines Agency (2004) Note for guidance on the evaluation of the pharmacokinetics of medicinal products in patients with impaired renal function. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003123.pdf. Accessed 11 Feb 2013
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2010) Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis and impact on dosing and labeling. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf. Accessed 08 Dec 2012
Pfistermeister B, Schenk C, Kornhuber J, Burkle T, Fromm MF, Maas R (2013) Different indications, warnings and precautions, and contraindications for the same drug–an international comparison of prescribing information for commonly used psychiatric drugs. Pharmacoepidemiol Drug Saf 22(3):329–333
Garbe E, Andersohn F (2007) Contraindication labelling changes in the United States and Germany. Eur J Clin Pharmacol 63(1):87–93
Arguello B, Fernandez-Llimos F (2007) Clinical pharmacology information in summaries of product characteristics and package inserts. Clin Pharmacol Ther 82(5):566–571
Rougemont M, Ulrich S, Hiemke C, Corruble E, Baumann P (2010) French summaries of product characteristics: content in relation to therapeutic monitoring of psychotropic drugs. Fundam Clin Pharmacol 24(3):377–384
Duke J, Friedlin J, Li X (2013) Consistency in the safety labeling of bioequivalent medications. Pharmacoepidemiol Drug Saf 22(3):294–301
Wall AJ, Bateman DN, Waring WS (2009) Variability in the quality of overdose advice in Summary of Product Characteristics (SPC) documents: gut decontamination recommendations for CNS drugs. Br J Clin Pharmacol 67(1):83–87
Fernandez-Llimos F (1999) Information on drugs for the community pharmacy. Pharm Care Esp 1:90–96
Bergk V, Haefeli WE, Gasse C, Brenner H, Martin-Facklam M (2005) Information deficits in the summary of product characteristics preclude an optimal management of drug interactions: a comparison with evidence from the literature. Eur J Clin Pharmacol 61(5–6):327–335
Salgado TM, Arguello B, Benrimoj SI, Fernandez-Llimos F (2012) Discordances in the classification of renal impairment in summaries of product characteristics. In: FIP Centennial Congress. Amsterdam
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3(1):1–150
Geerts AF, De Koning FH, Van Solinge WW, De Smet PA, Egberts TC (2012) Instructions on laboratory monitoring in 200 drug labels. Clin Chem Lab Med 50(8):1351–1358
Rahmner PB, Eiermann B, Korkmaz S, Gustafsson LL, Gruven M, Maxwell S, Eichle HG, Veg A (2012) Physicians’ reported needs of drug information at point of care in Sweden. Br J Clin Pharmacol 73(1):115–125
Perianez-Parraga L, Martinez-Lopez I, Ventayol-Bosch P, Puigventos-Latorre F, Delgado-Sanchez O (2012) Drug dosage recommendations in patients with chronic liver disease. Rev Esp Enferm Dig 104(4):165–184
McEvoy GK (2005) Dose adjustment in renal impairment: response from AHFS Drug Information. BMJ 331(7511):293
Schwartz LM, Woloshin S (2009) Lost in transmission–FDA drug information that never reaches clinicians. N Engl J Med 361(18):1717–1720
Vidal L, Shavit M, Fraser A, Paul M, Leibovici L (2005) Systematic comparison of four sources of drug information regarding adjustment of dose for renal function. BMJ 331(7511):263
Seidling HM, Al Barmawi A, Kaltschmidt J, Bertsche T, Pruszydlo MG, Haefeli WE (2007) Detection and prevention of prescriptions with excessive doses in electronic prescribing systems. Eur J Clin Pharmacol 63(12):1185–1192
Payne TH, Nichol WP, Hoey P, Savarino J (2002) Characteristics and override rates of order checks in a practitioner order entry system. Proc AMIA Symp:602–606
van der Sijs H, Aarts J, Vulto A, Berg M (2006) Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 13(2):138–147
Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS (2003) Physicians’ decisions to override computerized drug alerts in primary care. Arch Intern Med 163(21):2625–2631
Acknowledgements
We would like to thank Fundaçao para a Ciencia e a Tecnologia, Ministry of Education and Science, Portugal, for supporting this work (Doctoral Grant reference number SFRH/BD/43999/2008).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Online Appendix 1
Formulary structure used to extract data from SmPCs (DOC 41 kb)
ESM 2
(XLSX 69 kb)
Rights and permissions
About this article
Cite this article
Salgado, T.M., Arguello, B., Martinez-Martinez, F. et al. Clinical relevance of information in the Summaries of Product Characteristics for dose adjustment in renal impairment. Eur J Clin Pharmacol 69, 1973–1979 (2013). https://doi.org/10.1007/s00228-013-1560-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1560-2