Abstract
Background
Biological agents have been widely used in the treatment of Crohn’s disease (CD). These drugs carry the risk of excessive immunosuppression, indicating possible opportunistic infections including opportunistic viral infections, but no meta analysis has ever focused on this issue.
Purpose
To evaluate whether there is an association between biological agents therapy and the risk of opportunistic viral infections and serious infections in patients with CD.
Methods
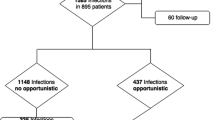
A search of online databases was performed and literature selection was carried out according to the inclusion and exclusion criteria by reading titles, abstracts and full texts. Study heterogeneity and publication bias were assessed. Whether to choose a fixed effects model or a random effects model depended on the result of heterogeneity test.
Results
There was a statistical significance in opportunistic viral infection events between the biological agents group and the placebo group. However, our analysis didn’t observe statistically significant differences between the two groups when combined analyses were carried out for herpes zoster and herpes simplex separately. A risk trend in the biological agents group was observed in the analysis for herpes zoster. More analyses aimed at the outcome measures and including influenza and serious infection were carried out separately, but no statistical significance was found in them.
Conclusion
Biological agents use might increase the risk of opportunistic viral infections in patients with CD, but not the risk of herpes simplex and serious infections. More randomized controlled trials (RCTs) are needed to draw the conclusion of whether they could elevate the risk of herpes zoster.




Similar content being viewed by others
References
Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP (2002) Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 8(4):244–250
Baumgart DC, Sandborn WJ (2012) Crohn’s disease. Lancet 380(9853):1590–1605
Gionchetti P, Calabrese C, Tambasco R, Brugnera R, Straforini G, Liguori G, Fornarini GS, Riso D, Campieri M, Rizzello F (2011) Role of conventional therapies in the era of biological treatment in Crohn’s disease. World J Gastroenterol 17(14):1797–1806
Burger D, Travis S (2011) Conventional medical management of inflammatory bowel disease. Gastroenterology 140(6):1827–1837
Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, Langholff W, Londhe A, Sandborn WJ (2012) Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol 107(9):1409–1422
Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel JF (2008) Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut 57:549–558
Lin Z, Bai Y, Zheng P (2011) Meta-analysis: efficacy and safety of combination therapy of infliximab and immunosuppressives for Crohn’s disease. Eur J Gastroenterol Hepatol 23(12):1100–1110
Higgins JPT, Green S (updated March 2011) Cochrane handbook for systematic reviews of interventions: version 5.1.0. www.handbook.cochrane.org
Ruemmele FM, Lachaux A, Cézard JP, Morali A, Maurage C, Giniès JL, Viola S, Goulet O, Lamireau T, Scaillon M, Breton A, Sarles J, Groupe Francophone d’Hépatologie, Gastroentérologie et Nutrition Pédiatrique (2009) Efficacy of infliximab in pediatric Crohn’s disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm Bowel Dis 15(3):388–394
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P, SONIC Study Group (2010) Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 362(15):1383–1395
Van Assche G, Vermeire S, Ballet V, Gabriels F, Noman M, D’Haens G, Claessens C, Humblet E, Vande Casteele N, Gils A, Rutgeerts P (2012) Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 61(2):229–234
Feagan BG, Sandborn WJ, Wolf DC, Coteur G, Purcaru O, Brabant Y, Rutgeerts PJ (2011) Randomised clinical trial: improvement in health outcomes with certolizumab pegol in patients with active Crohn’s disease with prior loss of response to infliximab. Aliment Pharmacol Ther 33(5):541–550
Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ (1997) A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. N Engl J Med 337(15):1029–1035
Rutgeerts P, D’Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, Mayer L, Van Hogezand RA, Braakman T, DeWoody KL, Schaible TF, Van Deventer SJ (1999) Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 117(4):761–769
Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ (1999) Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 340(18):1398–1405
Schreiber S, Colombel JF, Bloomfield R, Nikolaus S, Schölmerich J, Panés J, Sandborn WJ, PRECiSE 2 Study Investigators (2010) Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 105(7):1574–1582
Winter TA, Wright J, Ghosh S, Jahnsen J, Innes A, Round P (2004) Intravenous CDP870, a PEGylated Fab’ fragment of a humanized antitumour necrosis factor antibody, in patients with moderate-to-severe Crohn’s disease: an exploratory study. Aliment Pharmacol Ther 20(11–12):1337–1346
Sands BE, Kozarek R, Spainhour J, Barish CF, Becker S, Goldberg L, Katz S, Goldblum R, Harrigan R, Hilton D, Hanauer SB (2007) Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm Bowel Dis 13(1):2–11
Sandborn WJ, Feagan BG, Hanauer SB, Present DH, Sutherland LR, Kamm MA, Wolf DC, Baker JP, Hawkey C, Archambault A, Bernstein CN, Novak C, Heath PK, Targan SR, CDP571 Crohn’s Disease Study Group (2001) An engineered human antibody to TNF (CDP571) for active Crohn’s disease: a randomized double-blind placebo-controlled trial. Gastroenterology 120(6):1330–1338
Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Palmer T, Donoghue S, Natalizumab Pan-European Study Group (2003) Natalizumab for active Crohn’s disease. N Engl J Med 348(1):24–32
Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P, International Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) Trial Group, Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group (2005) Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 353(18):1912–1925
Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A, CDP870 Crohn’s Disease Study Group (2005) A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology 129(3):807–818
Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, Coteur G, Guzman JP, D’Haens GR (2011) Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol 9(8):670–678
Lémann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, Laharie D, Moreau J, Cadiot G, Picon L, Bourreille A, Sobahni I, Colombel JF, Groupe d’Etude Therapeutique des Affections Inflammatoires du Tube Digestif (GETAID) (2006) Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology 130(4):1054–1061
Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M, Wolf DC, Hernandez C, Bornstein J, Sandborn WJ, International Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) Trial Group (2007) Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology 132(5):1672–1683
Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF (2007) Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 132(1):52–65
Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, Li J, Pollack PF (2007) Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 56(9):1232–1239
Colombel JF, Schwartz DA, Sandborn WJ, Kamm MA, D’Haens G, Rutgeerts P, Enns R, Panaccione R, Schreiber S, Li J, Kent JD, Lomax KG, Pollack PF (2009) Adalimumab for the treatment of fistulas in patients with Crohn’s disease. Gut 58(7):940–948
Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel JF, Panaccione R, D’Haens G, Li J, Rosenfeld MR, Kent JD, Pollack PF (2007) Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 146(12):829–838
Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S, PRECISE 1 Study Investigators (2007) Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 357(3):228–238
Hanauer SB, Panes J, Colombel JF, Bloomfield R, Schreiber S, Sandborn WJ (2010) Clinical rial: impact of prior infliximab therapy on the clinical response to certolizumab pegol maintenance therapy for Crohn’s disease. Aliment Pharmacol Ther 32(3):384–393
Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P (2006) Human anti-tumor necrosis factor monoclonal antibody adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 130(2):323–333
Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ (2004) Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 350(9):876–885
Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P, ACCENT I Study Group (2002) Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 359(9317):1541–1549
Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ, PRECISE 2 Study Investigators (2007) Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 357(3):239–250
Schreiber S, Lawrance IC, Thomsen OØ, Hanauer SB, Bloomfield R, Sandborn WJ (2011) Randomised clinical trial: certolizumab pegol for fistulas in Crohn’s disease—subgroup results from a placebo-controlled study. Aliment Pharmacol Ther 33(2):185–193
Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, Johanns J, Blank M, Rutgeerts P, Ustekinumab Crohn’s Disease Study Group (2008) A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 135(4):1130–1141
Kimball AB, Gordon KB, Fakharzadeh S, Yeilding N, Szapary PO, Schenkel B, Guzzo C, Li S, Papp KA (2012) Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol 166(4):861–872
Hutchinson M, Kappos L, Calabresi PA, Confavreux C, Giovannoni G, Galetta SL, Havrdova E, Lublin FD, Miller DH, O’Connor PW, Phillips JT, Polman CH, Radue EW, Rudick RA, Stuart WH, Wajgt A, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, AFFIRM and SENTINEL Investigators (2009) The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 256(3):405–415
Probert CS, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, Arnott ID, Forbes A (2003) Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 52(7):998–1002
Choy EH, Hazleman B, Smith M, Moss K, Lisi L, Scott DG, Patel J, Sopwith M, Isenberg DA (2002) Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology 41(10):1133–1137
Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ (2008) Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 134(4):929–936
Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D’Inca R, Bossa F, Angelucci E, Biancone L, Gionchetti P, Ardizzone S, Papi C, Fries W, Danese S, Riegler G, Cappello M, Castiglione F, Annese V, Orlando A (2011) Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 9(1):30–35
Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, Targan SR (1997) A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol 159(12):6276–6282
van Deventer SJ (1999) Review article: targeting TNF alpha as a key cytokine in the inflammatory processes of Crohn’s disease–the mechanisms of action of infliximab. Aliment Pharmacol Ther 13(Suppl 4):3–8
Lobb RR, Hemler ME (1994) The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest 94(5):1722–1728
Rüegg C, Postigo AA, Sikorski EE, Butcher EC, Pytela R, Erle DJ (1992) Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol 117(1):179–189
Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC (1993) Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74(1):185–195
Utsugi M, Dobashi K, Ono A, Ishizuka T, Matsuzaki S, Hisada T, Shimizu Y, Kawata T, Aoki H, Kamide Y, Mori M (2009) PI3K p110beta positively regulates lipopolysaccharide-induced IL-12 production in human macrophages and dendritic cells and JNK1 plays a novel role. J Immunol 182(9):5225–5231
Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH (1998) The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 16:495–521
Hall MA, McGlinn E, Coakley G, Fisher SA, Boki K, Middleton D, Kaklamani E, Moutsopoulos H, Loughran TP Jr, Ollier WE, Panayi GS, Lanchbury JS (2000) Genetic polymorphism of IL-12 p40 gene in immune-mediated disease. Genes Immun 1(3):219–224
Stallmach A, Marth T, Weiss B, Wittig BM, Hombach A, Schmidt C, Neurath M, Zeitz M, Zeuzem S, Abken H (2004) An interleukin 12 p40-IgG2b fusion protein abrogates T cell mediated inflammation: anti-inflammatory activity in Crohn’s disease and experimental colitis in vivo. Gut 53(3):339–345
Kamada N, Hisamatsu T, Honda H, Kobayashi T, Chinen H, Takayama T, Kitazume MT, Okamoto S, Koganei K, Sugita A, Kanai T, Hibi T (2010) TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn’s disease. Inflamm Bowel Dis 16(4):568–575
Sarra M, Pallone F, Macdonald TT, Monteleone G (2010) IL-23/IL-17 axis in IBD. Inflamm Bowel Dis 16(10):1808–1813
Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F (2010) Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33(2):279–288
Acknowledgments
This study is supported by research grants from the Public health project of China (No. 201002020).
Conflicts of interest
The authors declare that they have nothing to disclose.
Authorship
Guarantor of the article: Bing Xia.
Author contributions: All the authors contributed to the design of the study. Xiaobing Wang, Junzhang Zhao were responsible for writing of the manuscript. Xiaobing Wang, Rui Zhou, Meifang Huang, Jin Li and Shufang Xu took part in the literature search and quality assessment and the data extraction. Xiaobing Wang and Wei Wang performed the statistical analysis. All authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaobing Wang, Feng Zhou and Junzhang Zhao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM FigureS1
The forest plot of randomized controlled trials of biological agents versus placebo in herpes zoster events in CD (JPEG 47 kb)
ESM FigureS2
The forest plot of randomized controlled trials of biological agents versus placebo in herpes simplex events in CD (JPEG 51 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Zhou, F., Zhao, J. et al. Elevated risk of opportunistic viral infection in patients with Crohn’s disease during biological therapies: a meta analysis of randomized controlled trials. Eur J Clin Pharmacol 69, 1891–1899 (2013). https://doi.org/10.1007/s00228-013-1559-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1559-8