Abstract
Aim
To develop and validate an algorithm for the prediction of therapeutic dose of acenocoumarol in Romanian patients.
Methods
The inclusion criteria for entry to the study was age ≥18 years and starting acenocoumarol treatment for at least one of the following clinical indications: acute deep vein thrombosis of the lower limbs, persistent or permanent atrial fibrillation, and/or the presence of valvular prostheses requiring prolonged oral anticoagulant therapy. The patients were followed up for 3 months. Patients admitted to the internal medicine, cardiology, and geriatrics wards of the Municipal Clinical Hospital, Cluj-Napoca and “Niculae Stăncioiu” Heart Institute between October 2009 and June 2011 who fulfilled the inclusion criteria were included in the study. Clinical and demographic data that could influence the acenocoumarol stable dose were recorded for each patient. Genetic analysis included the genotyping the CYP2C9*2 and *3, and the VKORC1 -1693 G > A polymorphisms. The patients were randomly divided into two groups: (1) the main group on which the development of the clinical and genetic algorithms for acenocoumarol dose prediction was based; (2) the validation group.
Results
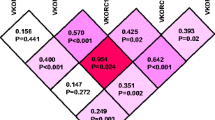
The study included 301 patients, of whom 155 were women (51.5 %) and 146 were men (48.5 %). The median age of the patient cohort was 66 (women, 57; men, 73) years. After randomization the main group comprised 200 patients (66.4 %) and the validation group 101 patients (33.6 %). Age and body mass index explained 18.8 % (R 2) of the variability in acenocoumarol weekly dose in patients in the main group. When the genetic data were added to the algorithm, the CYP2C9*2 and *3 polymorphisms and the VKORC1 -1693 G > A polymorphism accounted for 4.7 and 19. 6 % of acenocoumarol dose variability, respectively. For the main group, we calculated a mean absolute error of 5 mg/week (0.71 mg/day). In the validation group, clinical parameters explained 22.2 % of the weekly acenocoumarol dose variability. Genetic polymorphisms increased the R 2 coefficient to 32.8 %.
Conclusion
We have developed and validated an accurate algorithm for prediction of the stable therapeutic dose of acenocoumarol in a Romania population.
Similar content being viewed by others
References
Takahashi H, Wilkinson GR, Padrini R, Echizen H (2004) CYP2C9 and oral anticoagulation therapy with acenocoumarol and warfarin: similarities yet differences. Clin Pharmacol Ther 75(5):376–380. doi:10.1016/j.clpt.2004.01.007
Zhou SF, Liu JP, Chowbay B (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41(2):89–295. doi:10.1080/03602530902843483
Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G (2012) Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th edn: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141[2 Suppl]:e44S–e88S. doi:10.1378/chest.11-2292
Buzoianu AD, Trifa AP, Mureşanu DF, Crişan S (2012) Analysis of CYP2C9*2, CYP2C9*3 and VKORC1–1639 G > A polymorphisms in a population from South-Eastern Europe. J Cell Mol Med 16(12):2919–2924. doi:10.1111/j.1582-4934.2012.01606.x
Yin T, Miyata T (2007) Warfarin dose and the pharmacogenomics of CYP2C9 and VKORC1—rationale and perspectives. Thrombosis Res 120(1):1–10. doi:10.1016/j.thromres.2006.10.021
Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR (1994) Impaired S-warfarin metabolism catalyzed by R144C allelic variant of CYP2C9. Pharmacogenetics 4:39–42
Crespi CL, Miller VP (1997) The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH:cytochrome P450 oxidoreductase. Pharmacogenetics 7:203–210
Schalekamp T, Brassé BP, Roijers JFM, Chahid Y, van Geest-Daalderop JHH et al (2006) VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: Interaction between both genotypes affects overanticoagulation. Clin Pharmacol Ther 80(1):13–22. doi:10.1016/j.clpt.2006.04.006
Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM et al (2002) Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287(13):1690–1698. doi:10.1001/jama.287.13.1690
Visser LE, van Vliet M, van Schaik RH, Kasbergen AA, De Smet PA, Vulto AG et al (2004) The risk of overanticoagulation in patients with cytochrome P450 CYP2C9*2 or CYP2C9*3 alleles on acenocoumarol or phenprocoumon. Pharmacogenetics 14(1):27–33
Cadamuro J, Dieplinger B, Felder T, Kedenko I, Mueller T, Haltmayer M et al (2010) Genetic determinants of acenocoumarol and phenprocoumon maintenance dose requirements. Eur J Clin Pharmacol 66(3):253–260. doi:10.1007/s00228-009-0768-7
Goodstadt L, Ponting CP (2004) Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends Biochem Sci 29(6):289–292. doi:10.1016/j.tibs.2004.04.004
Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG et al (2006) Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics 16(2):101–110. doi:10.1097/01.fpc.0000184955.08453.a8
Montes R, Ruiz de Gaona E, Martínez-González MA, Alberca I, Hermida J (2006) The c.-1639G > A polymorphism of the VKORC1 gene is a major determinant of the response to acenocoumarol in anticoagulated patients. Br J Haematol 133(2):183–187. doi:10.1111/j.1365-2141.2006.06007.x
Harrison L, Johnston M, Massicotte MP, Crowther M, Moffat K, Hirsh J (1997) Comparison of 5-mg and 10-mg loading doses in initiation of warfarin therapy. Ann Intern Med 126(2):133–136. doi:10.7326/0003-4819-126-2-199701150-00006
Kovacs MJ, Rodger M, Anderson DR, Morrow B, Kells G, Kovacs J et al (2003) Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial. Ann Intern Med 138(9):714–719
Joffe HV, Xu R, Johnson FB, Longtine J, Kucher N, Goldhaber SZ (2004) Warfarin dosing and cytochrome P450 2C9 polymorphisms. Thromb Haemost 91(6):1123–1128. doi:10.1160/TH04-02-0083
Brockmöller J, Tzvetkov MT (2008) Pharmacogenetics: data, concepts and tools to improve drug discovery and drug treatment. Eur J Clin Pharmacol 64(2):133–157. doi:10.1007/s00228-007-0424-z
Stehle S, Kirchheiner J, Lazar A, Fuhr U (2008) Pharmacogenetics of oral anticoagulants: a basis for dose individualization. Clin Pharmacokinet 47(9):565–594. doi:10.2165/00003088-200847090-00002
Borobia AM, Lubomirov R, Ramírez E, Lorenzo A, Campos A, Muñoz-Romo R et al (2012) An acenocoumarol dosing algorithm using clinical and pharmacogenetic data in spanish patients with thromboembolic disease. PLoS One 7(7):e41360. doi:10.1371/journal.pone.0041360
Verde Z, Ruiz JR, Santiago C, Valle B, Bandrés F et al (2010) A novel, single algorithm approach to predict acenocoumarol dose based on CYP2C9 and VKORC1 allele variants. PLoS One 5(6):e11210. doi:10.1371/journal.pone.0011210
van Schie RMF, Wessels JAM, le Cessie S, de Boer A, Schalekamp T, van der Meer FJM et al (2011) Loading and maintenance dose algorithms for phenprocoumon and acenocoumarol using patient characteristics and pharmacogenetic data. Eur Heart J 32(15):1909–1917. doi:10.1093/eurheartj/ehr116
Aynacioglu AS, Brockmöller J, Bauer S, Sachse C, Güzelbey P, Ongen Z et al (1999) Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol 48(3):409–415. doi:10.1046/j.1365-2125.1999.00012.x
Wen MS, Lee M, Chen JJ, Chuang HP, Lu LS, Chen CH et al (2008) Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther 84(1):83–89. doi:10.1038/sj.clpt.6100453
Arboix M, Laporte JR, Frati ME, Rutllan M (1984) Effect of age and sex on acenocoumarol requirements. Br J Clin Pharmacol 18(4):475–479
Loi CM, Vestal RE (1988) Drug metabolism in the elderly. Pharmacol Ther 36:131–149. doi:10.1016/0163-7258(88)90115-5
Tanaka E (1998) In vivo age-related changes in hepatic drug-oxidizing capacity in humans. J Clin Pharm Ther 23(4):247–255
Dreisbach AW, Lertora JJ (2008) The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol 4(8):1065–1074. doi:10.1517/17425255.4.8.1065
Husted S, Andreasen F (1977) The influence of age on the response to anticoagulants. Br J Clin Pharmacol 4:559–565
Teichert M, van Noord C, Uitterlinden AG, Hofman A, Buhre PN, De Smet PA et al (2011) Proton pump inhibitors and the risk of overanticoagulation during acenocoumarol maintenance treatment. Br J Haematol 153(3):379–385. doi:10.1111/j.1365-2141.2011.08633.x
Vreeburg EM, De Vlaam-Schluter GM, Trienekens PH, Snel P, Tytgat GN (1997) Lack of effect of omeprazole in oral acenocoumarol anticoagulant therapy. Scand J Gastroenterol 32(10):991–994. doi:10.3109/00365529709011215
de Hoon JN, Thijssen HH, Beysens AJ, Van Bortel LM (1997) No effect of short-term omeprazole intake on acenocoumarol pharmacokinetics and pharmacodynamics. Br J Clin Pharmacol 44(4):399–401. doi:10.1046/j.1365-2125.1997.00600.x
van Schie RM, Verhoef TI, Boejharat SB, Schalekamp T, Wessels JA, le Cessie S et al (2012) Evaluation of the effect of statin use on the acenocoumarol and phenprocoumon maintenance dose. Drug Metabol Drug Interact 27(4):229–234. doi:10.1515/dmdi-2012-0024
Acknowledgments
This study was supported by research grant 42-127/2008 “Trombo-Gen”, from National Authority for Scientific Research from Romania.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 21.8 kb)
Rights and permissions
About this article
Cite this article
Pop, T.R., Vesa, Ş.C., Trifa, A.P. et al. An acenocoumarol dose algorithm based on a South-Eastern European population. Eur J Clin Pharmacol 69, 1901–1907 (2013). https://doi.org/10.1007/s00228-013-1551-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1551-3